A Rice University bioengineering team has developed a technique to generate tunable electrospun scaffolds completely derived from decellularized skeletal muscle. The discovery will promote the regeneration of injured skeletal muscle and could significantly enhance patient healing associated with an estimated 4.5 million reconstructive surgeries that take place each year in the United States.
In a paper published by Science Advances, the bioengineers revealed how a natural extracellular matrix can be manipulated to take on the role of native skeletal muscle. The technique promotes the alignment and growth of myotubes, an essential skeletal muscle element.
While multiple methods for creating tunable electrospun scaffolds currently exist, the Rice University approach involves important differences, according to lead author Mollie Smoak.
“The method we developed allows us to fabricate highly tunable electrospun scaffolds completely derived from decellularized skeletal muscle. Most other systems use a blend of decellularized extracellular matrix and a synthetic polymer,” Smoak said. “Our system more closely mimics the degradation kinetics needed to temporally break down as new skeletal muscle grows. In addition, the biomaterial releases pro-regenerative degradation products that can further drive cell differentiation.”


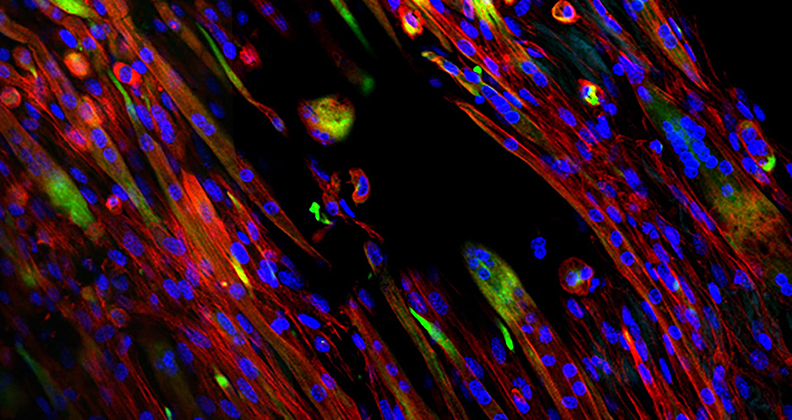
Samples of electrospun decellularized skeletal muscle extracellular matrix created by Rice University bioengineers. Such scaffolds can be used to regenerate injured muscles. The natural scaffold material degrades as new muscles take over.
Citing the discovery’s potential impact on orthopedic medicine, Smoak said the proprietary method promoted tunable material properties and retained bioactivity throughout the processes of decellularization and electrospinning.
“We found that we could control cell growth and myotube differentiation and alignment by modulating fiber alignment and crosslinking density,” Smoak said. “Even with a natural material, we were able to promote a very high level of myotube alignment, which is crucial for skeletal muscle engineering.”
She went on to say that she and her team hope to deliver muscle-specific biochemical cues and provide mechanical and topographical stimuli similar to native muscle to repair skeletal muscle following injury.
“We also believe that this platform of electrospinning decellularized extracellular matrix has broad applications in supporting other soft tissue repair,” she added.
Through the use of bioactive cues from decellularized muscle with the tunable material properties, a material with sophisticated biochemical signals and highly specific topography is created. The material is designed to break down gradually as it is replaced by new muscle.
“The use of decellularized extracellular matrix has been used for many years and has been clinically approved for a number of clinical applications,” said Smoak, who noted that traditional synthesized methods come with the major drawback of cell growth being difficult to predict and control. “We hope that the platform that we have developed to electrospin decellularized tissues can be adapted for a number of tissue engineering applications, including skeletal muscle regeneration.”
PM
Patrick McGuire is a BONEZONE Contributor.




