Repairing rotator cuff tears is a significant clinical challenge. The overall failure rate ranges from 20% to 90%, with the larger the initial tear, the higher the risk of it tearing again after surgery.
“In order to design devices that address re-tear rates, you first need to understand the cause,” said Saif Khalil, Ph.D., Chairman, CEO and a founder of Aevumed, which offers a line of Phantom anchors designed to improve rotator cuff repairs.
Broadly speaking, the causes can be grouped into two main categories: mechanical issues related to the implant used to make the repair, and biological factors that prevent optimal healing at the repair site.
From a mechanical standpoint, surgeons face several challenges with current technologies and surgical techniques. One key issue is the ability to properly re-tension the suture used to reattach the rotator cuff tendon to the humerus.
“This is critical because if the suture is too loose, the tendon isn’t adequately fixed to the bone,” Dr. Khalil said. “Any motion at that site can prevent healing.”
If the suture is too tight, however, it can restrict blood flow. The tendon is already relatively avascular to begin with, so further compromising its blood supply can significantly impact its ability to reattach to bone.
That’s why surgeons seek solutions that allow for adjustable suture tensioning during surgery, after the repair is in place.
The other mechanical issue relates to the design of anchors used in the repair, particularly in double-row techniques. Most of the anchors currently on the market involve positioning the sutures on the outside of the anchor.
“In patients with soft or osteoporotic bone, these exposed sutures can cut through the bone over time,” Dr. Khalil said. “Because they sit on the outside of the anchor, the stress isn’t well distributed, leading to suture cutout or pull-through.”
Surgeons want anchors and repair systems that allow for re-tensioning and reduce the risk of suture-related bone damage, especially in softer bone conditions, Dr. Khalil said.
Aevumed’s Phantom-LP was designed specifically to address these mechanical challenges. It’s the first 100% PEEK anchor that is fully seated into the bone before surgeons tension the suture. More importantly, surgeons can adjust the tension during surgery and relock the device in place to optimize the repair’s stability and strength.
Phantom-LP’s Bone Shield technology contains the sutures inside the anchor. This design factor is critical for patients with softer bone, because it helps to prevent the “cheese-cutting effect,” in which sutures can cut through the bone.
Avoiding this complication prevents loosening of the suture that could compromise tendon fixation and helps maintain the biomechanical pullout strength of the anchor, reducing the risk of repair failure.
PEEK can also improve the anchor performance in rotator cuff repairs by reducing the risk of stress shielding. Dr. Khalil explained that PEEK has an elastic modulus of about 4 GPa, which is similar to natural bone. The material is flexible under stress, leading to a more physiological distribution of forces. This reduces the risk of stress shielding and promotes better bone maintenance around the implant.
While PEEK is generally bioinert, certain surface treatments — like micro-texturing or coating — can enhance bone apposition. “But the real mechanical advantage lies in its modulus, and how well it works with the bone rather than against it,” Dr. Khalil said.
Helping the Healing Process
The health of the torn rotator cuff tendon is often compromised, especially in patients with large tears. If the patient is diabetic, a smoker or dealing with other systemic health issues, healing becomes even more difficult.
“The biologic side of repair involves introducing biomaterials that can support and enhance the healing process,” Dr. Khalil said. “One of the most effective materials for soft tissue, particularly tendons, is collagen.”
There are several collagen-based rotator cuff repair patches on the market that were originally designed for partial-thickness tears.
“In those cases, you don’t necessarily need anchors or sutures,” Dr. Khalil said. “The surgeon applies a patch directly over the tendon to help stimulate healing and reinforce a weak area before it fully fails.”
But the primary challenge is reattaching the tendon to the bone — that’s the critical interface where true healing must
occur — and in most patch-based solutions, the material is applied on top of the repair, not at the tendon/bone junction.
“Patches might offer surface reinforcement of the repair site, but they’re not necessarily enhancing healing where it matters most,” Dr. Khalil said. “Healing does occur on the top of the tendon, but the critical interface between the tendon and bone is not addressed.”
Aevumed’s Phantom-X addresses that healing step. The 3D printed device is designed to be anatomically specific, not patient-specific; it’s built specifically for the rotator cuff anatomy and engineered with variable thickness.
Phantom-X is thicker on the top, where it provides structural support, and extremely thin on the bottom, which allows it to conform closely to the bone/tendon interface. “That thin bottom layer is critical,” Dr. Khalil said. “It doesn’t create too much separation between the tendon and bone, which could otherwise inhibit healing.”
The single-scaffold design wraps around the rotator cuff, supporting it from above and below. “To our knowledge, this is the first solution designed specifically to cover both surfaces of the tendon in this way,” Dr. Khalil said. “Other products typically cover either the top or the bottom, but not both.”
Phantom-X is not yet FDA cleared. Proof of concept has been completed, and surgeons have reported positive results in lab testing.
Anika Therapeutics fully introduced the Integrity Implant System last year. The esterified hyaluronic acid (HA)-based scaffold is a regenerative implant designed to treat tendon augmentation and repair. It’s intended for rotator cuff repair and other tendon applications.
Integrity is a knitted scaffold that combines HA fibers with a small percentage of polyethylene terephthalate (PET), the same material used in high-strength sutures. PET gives Integrity added mechanical strength and durability, while the HA component provides biological performance. The knitted construction gives it flexibility and mechanical resilience, even in wet surgical environments.
“These features create a scaffold that’s strong, conformable and biologically active, without compromising on handling or ease of use,” said Cheryl Blanchard, Ph.D., Anika’s CEO.
Integrity’s mechanical strength is a critical differentiator, according to Dr. Blanchard. “Unlike first generation collagen-based patches, which tend to weaken dramatically when wet, Integrity retains its suture retention strength, making it easy for surgeons to manipulate and implant,” she said.
In preclinical testing, Integrity demonstrated a significant regenerative response driven by the HA component, which provides a bioactive environment for healing and tissue integration.
“Our primary goal in designing Integrity was to create a biologically active solution that supports the healing process,” said orthopedic surgeon Christopher Baker, M.D., who consults for Anika and performed the first surgeries involving Integrity at the Florida Orthopaedic Institute in Tampa. “HA is naturally found throughout the body. It’s safe, familiar and already widely used in orthopedic applications, especially in cartilage and tendon repair.”
That biological profile was critical, according to Dr. Baker.
“Most similar grafts and implants on the market are made from animal-derived collagen, typically bovine,” he said. “Many biologic implants don’t offer much mechanical support immediately after placement. Even if they perform well biologically, they might fail to provide the reinforcement needed right away. We knew we needed something that could promote healing and hold up structurally.”
Anika continues to innovate the Integrity platform by developing various shapes, sizes and configurations tailored to a wider range of tendon repair anatomies. New product launches are planned for later this year.
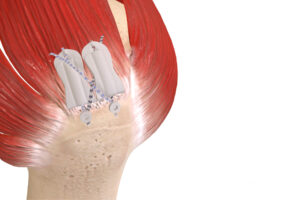
Atreon Orthopedics designed BioCharge to address biological failure in rotator cuff repair.
Synthetic Solution
In February, Atreon Orthopedics received FDA 510(k) clearance for the BioCharge Autobiologic Matrix, a bioresorbable synthetic implant designed to address biological failure in rotator cuff repair while improving repair integrity. Atreon’s electrospun nanofiber platform technology distinguishes itself from traditional augmentarion devices, which rely on animal-processed collagen or human dermal allografts.
“Consistency matters in rotator cuff repair,” said Steven Ogg, Vice President of Business Development at Atreon. “Whether it’s the first implant or the millionth, our material contains the same formulation and polymer chemistry, and performs the same. That’s simply not possible with biologic grafts, which have inherent variability.”
Ogg said collagen-based products degrade slowly, typically over 52 to 56 weeks.
“In contrast, our implant resorbs in just 3 to 4 months, aligning much more closely with the behavior of native extracellular matrix,” he added. “Our resorbable scaffold is based on PGA and PLCL, polymers with well-understood, tunable degradation profiles. These materials do more than just disappear — they actively participate in the healing process by supporting early cell signaling, modulating inflammation and transitioning the tissue environment into a proliferative, regenerative state. That’s a huge advantage.”
Atreon spent more than 10 years developing the technology before commercializing it. “It’s not just another patch,” Ogg said. “It’s a thoughtfully engineered solution, backed by real science and built for consistent performance.”
The company’s Level I prospective study of the BioCharge technology is set to be published this year. A subset of the study’s patients showed a 93% success rate and a significant reduction in re-tear rates.
“Typically, re-tear rates for small to medium rotator cuff tears range from 35% to 40%,” Ogg said. “In our study, we’re seeing re-tear rates around 7% to 8%, which is a substantial improvement.”
Ogg said BioCharge results in early healing, but noted that repair failures can occur well beyond the initial recovery, even up to two years post-op.
“A patient might feel fine for a year or more, but if that initial healing didn’t happen properly, long-term outcomes can be compromised,” he explained. “That’s why our approach considers long-term outcomes.”
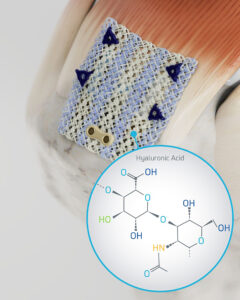
The Integrity System from Anika Therapeutics is a hyaluronic acid-based implant with bone and tendon fixation components.
Ease of Use
Dr. Khalil said traditional rotator cuff scaffolds take about 30 minutes to implant. “The surgeon needs to open the scaffold, manually thread sutures through each of the four corners and use a grasper to position it,” he added. “It’s a tedious, time-consuming process.”
Surgeons who were consulted during the development of Phantom-X suggested the implant should have a unique, integrated delivery mechanism. The pre-loaded, jaw-like device comes fully assembled. The scaffold is already in place, and the sutures and needles are integrated into the system.
“The surgeon needs to only open a sterile peel pack, position the scaffold and deploy it,” Dr. Khalil said. “There’s no need to pass sutures manually, and no extra steps. The entire process takes two to three minutes to complete.”
Anika paired the Integrity Implant with dedicated fixation tools and a simple, lateral surgical technique, making it fast and intuitive.
“This ease of use, combined with Integrity’s strength and biological activity, is driving significant interest among surgeons,” Dr. Blanchard said. “It’s our fastest growing product, with sequential quarter-over-quarter growth of 40%. We anticipate strong continued adoption, particularly in the U.S. market.”
Dr. Baker understands the importance of incorporating ease of use into a device’s design. “Integrity was made for quick placement,” he said. “In my own surgeries, it adds less than five minutes to the procedure. That’s a major win in terms of efficiency and workflow.”
Dr. Baker said Integrity is all about simplicity and reproducibility for surgeons.
“Surgeons fix the rotator cuff graft laterally, directly to the bone and just outside the repair site,” he said. “That way, when they’re finished with the augmentation, the graft ends up exactly where intended. There’s no drifting or repositioning — it’s locked in from the start.”
Additionally, no extra steps are needed to punch holes or place guides. “The entire implant goes in with a single-step insertion system,” Dr. Baker said. “It’s preloaded with the graft and an anchor. You simply punch it into the bone, and the graft is in place. There’s no fumbling with separate tools, no searching for pilot holes. It’s designed to be as streamlined and surgeon friendly as possible.”
Developing rotator cuff repair solutions that surgeons like to use is part of larger efforts to introduce devices that enhance healing for a surgery that still has plenty of room for improvement. It’s clear that significant opportunities exist for companies that work to fill the gap between optimal patient outcomes and current realities.
DC
Dan Cook is a Senior Editor at ORTHOWORLD. He develops content focused on important industry trends, top thought leaders and innovative technologies.




