
PEEK (Polyetheretherketone) has several advantages for healthcare applications. It exhibits high strength, durability, and impact resistance, making it suitable for load-bearing implants. PEEK is also radiolucent, which improves visibility in radiographic imaging, making it an optimal choice for implants that require post-operative monitoring. PEEK is also biocompatible and chemically resistant, so it is well-tolerated by the human body. Finally, its thermoplastic behavior simplifies processing and customization, making it a great alternative to metal for patient-specific implants.
While the material is well known within the orthopedic market, most PEEK implants are still produced using conventional manufacturing methods—like milling, turning, or injection molding—due to misconceptions about the ability to 3D-print PEEK and maintain its mechanical properties. The EXT 220 MED, 3D Systems’ proprietary filament-based extrusion technology addresses these challenges, enabling manufacturers to produce high-strength implants that meet ASTM standards for mechanical properties and performance.
This new, reliable way to fabricate with PEEK using 3D printing offers several advantages over traditional manufacturing, including the design of complex geometries with integrated lattice structures or porous scaffolds. These structures mimic trabecular bone and support bone ingrowth, facilitating osseointegration and long-term implant stability. Additionally, with cementless options in applications, device manufacturers can minimize post-processing steps and reduce material utilization.
What sets this technology apart?
Fully controllable build chamber heating. The platform uses a patented laminar airflow for homogeneous temperature distribution (up to 250°C) to prevent warping and increase layer adhesion. It also supports reproducibility by eliminating temperature gradients and adapting the temperature to specific thermogenic polymers like PEEK and Radel® PPSU.
Adaptive local temperature management. Temperature controls at the nozzle enable local cooling for each strand and layer individually or additional heat to further increase layer adhesion. This capability can reduce the need for post-processing and simplify the removal of support structures.
Integrated clean room. In addition to having the ability to connect to external clean room exhaust air systems, the EXT 220 MED has an internal filter system and creates a contamination-free build chamber, fulfilling ISO class 7 clean room requirements according to DIN EN ISO 14644.


EXT 220 MED Extrusion 3D Printer
Seamless data management. The system’s PLC (programmable logic controller) system and software monitoring process print parameters and record process analytics for regulatory compliance.
Designed for accuracy. Delta kinematics help to improve printing speed and precision, facilitating quick directional changes for smoother, intricate devices.
Fast printing speeds and minimal post-processing. The EXT 220 MED prints PEEK parts in minutes to a couple of hours, allowing for delivery of most implants in as little as two days from scan to surgery. Plus, with its latest performance upgrade, build times are up to 30% faster.
What are the latest 3D-printed orthopedic applications in PEEK?
PEEK is being used in a variety of orthopedic devices, including:
Spinal implants. It’s commonly used in interbody fusion cages, posterior cervical plates, and pedicle screws for spinal fusion surgeries.


Spinal Bone Plate in Carbon Fiber-Reinforced PEEK (CFR-PEEK)
Trauma implants. It’s an ideal material in trauma cases for applications, such as plates and screws, for fracture fixation.
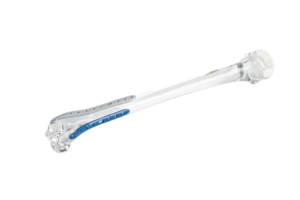

Trial Implants: Trauma Plates in Radel® PPSU
Extremity implants. It’s an option for use in hand, foot, and shoulder implants.


Knee Bone Plates with High Tibial Osteotomy Wedge
Want to discover more about the EXT 220 MED?
Visit 3dsystems.com/EXT-220 and fill out the online form, or contact your 3D Systems representative to start the conversation. Find out how this game-changing technology can help you create complex geometries, provide more design flexibility, reduce material utilization, shorten post-processing timelines and more.
Markus Reichmann, Director, Global Sales – Manufacturing Solutions, 3D Systems
Markus Reichmann is a passionate leader at the forefront of revolutionizing healthcare manufacturing solutions at 3D Systems. Markus and his team are on a mission to push the boundaries of what’s possible in the world of medical devices using additive manufacturing. They collaborate closely with Original Equipment Manufacturers and Contract Manufacturers to expand the scope of medical device applications using the latest technologies in 3D printing. Markus is thrilled to participate in this exciting movement and is determined to help medical device companies take the lead in bringing the next generation of 3D-printed devices to the market.
With his extensive experience in the industry, Markus joined 3D Systems in 2017. He worked closely with the company’s metals R&D group for the first three years at its Customer Innovation Center in Leuven, Belgium. In 2019 he moved to Littleton, Colorado, to focus on Business Development, where he continues to drive innovation and change. Markus is a graduate of the University of Applied Sciences in Furtwangen, Germany, where he obtained his bachelor’s degree in Mechanics and Mechatronics. He recently completed his Executive Master of Business Administration at Leeds School of Business at the University of Colorado Boulder in April 2023, which has helped him hone his leadership and management skills to lead his team to success.




