
While every year it seems that spinal products win the race of most clearances swept over all orthopedic market segments, enabling technologies are steadily gaining ground. Gyder Surgical, LEM Surgical and MSKai all announced first FDA 510(k) clearances in 2025 for systems that support spine and hip procedures.
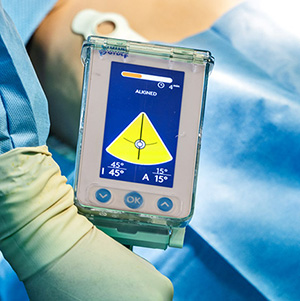 Gyder Surgical | GYDER Hip System, K243387
Gyder Surgical | GYDER Hip System, K243387
Submitted October 2024, granted January 2025
Primary predicate: OrthAlign Plus in the HipAlign configuration for Total Hip Arthroplasty, K130387
FYI: GYDER Hip is claimed to be the world’s first commercially available non-invasive (pin-less) and imageless solution for the accurate positioning of the acetabular cup during anterior hip arthroplasty.
Unlike traditional navigation approaches, GYDER Hip does not use metallic pins or rely on pre- or intra-operative imaging for anatomical landmark registration. One-minute calibration and quick registration support rapid computer-assisted navigation that integrates into existing surgical workflows. The system is suitable for ambulatory surgery center/outpatient use.
GYDER Hip received Australia’s TGA regulatory approval in 2023. Surgical cases involving the system have already occurred in Australia and India.
Gyder Surgical was founded in 2017 and is based in Australia with an office in California. Leadership brings experience from roles such as Vice President, Ambulatory Surgery Centers & Enabling Technologies at Johnson & Johnson.
The company has raised $1.8 million of its $3.7 million goal as of May 2024.
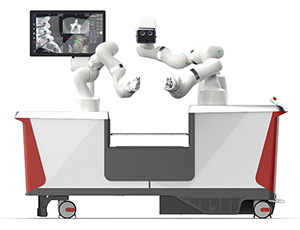 LEM Surgical | Dynamis Robotic Surgical System, K243326
LEM Surgical | Dynamis Robotic Surgical System, K243326
Submitted October 2024, granted April 2025
Primary predicate: Globus Medical, ExcelsiusGPS, K171651
FYI: Dynamis is an integrated navigation-based robotic platform designed to enhance accuracy and control during spine surgeries. The next-generation technology combines real-time imaging, dynamic guidance and adaptable instrumentation compatibility within a single platform.
It will be introduced in select U.S. hospitals this year, followed by increased commercialization in 2026.
The system features three robotic arms — two for surgical guidance and one for optical navigation — consolidated into a single cart that fits partially beneath the surgical table. Dynamis supports a range of surgical instruments through its adjustable end-effectors and intraoperative qualification process.
LEM was founded in 2021 and is based in Switzerland. The company has raised $37.1 million over Series A and B rounds, and leadership/staff brings experience from roles at Alphatec Spine, Mazor Robotics, Medtronic and Surgalign, among others
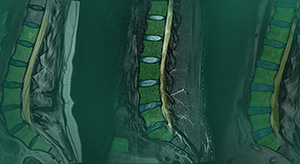 MSKai | Spine Imaging Software, K240793
MSKai | Spine Imaging Software, K240793
Submitted March 2024, granted December 2024
FYI: MSKai is a tool that assists qualified medical professionals in the evaluation of previously acquired T2-weighted lumbar spine MRIs. The software enables users to perform anatomy segmentation, labeling, measurement and export of quantitative and qualitative results into customizable reports. By delivering objective, repeatable spine measurements, MSKai supports greater efficiency and consistency in clinical imaging workflows.
MSKai is not a diagnostic device and does not provide or recommend a medical diagnosis or treatment. Instead, it serves as a decision-support tool that offers clear, repeatable insights for users trained in medical imaging. Users must confirm preferences, verify automated measurements and finalize reports in accordance with clinical best practices. The software was reviewed/cleared by FDA’s radiology panel.
Founded in 2021 and is based in Memphis, Tennessee, MSKai has raised $1 million to date over the course of two seed rounds.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




