Osteoarthritis (OA) affects 32.5 million adults in the U.S., with an overall economic burden of $137 billion annually, according to estimates by U.S. Centers for Disease Control and Prevention. The economic burden has more than doubled over the last decade and the number of impacted adults in the U.S. could double by 2040.
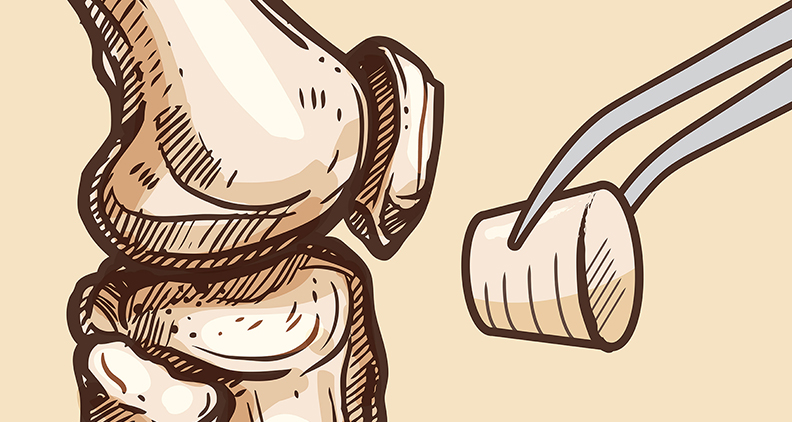
Osteochondral defects (OCDs) can damage the knee’s cartilage and underlying bone, resulting in chronic pain and impaired joint function. Depending on the severity of the damage, a total knee replacement might be necessary. Each year, more than 800,000 Americans undergo this procedure.
Early Treatment Option
Melissa Grunlan, Ph.D., Professor in the Department of Biomedical Engineering at Texas A&M University, has joined numerous companies and academics who are working to provide OA treatments that address the condition proactively.
Her research team received a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, a sub-organization of the National Institutes of Health (NIH), to develop synthetic cartilage-capped regenerative osteochondral plugs (CC-ROPs) that could prevent or delay the need for autografting or knee replacement surgery.
“Chronic knee pain and disability are caused by cartilage loss and OCDs stemming from arthritis, including osteoarthritis and post-traumatic arthritis causing individuals pain, that limits comfort and mobility,” Dr. Grunlan said. “The NIH grant will allow us to maximize the potential of CC-ROPs to heal OCDs, and to collect data demonstrating this efficacy.”
Her research group is broadly focused on developing new medical devices through innovations in materials, specifically polymers. “In the case of osteochondral lesions, biological autografts — or tissue plugs comprised of cartilage and underlying bone removed from other parts of the knee — are often used,” she said. “We thought about ways to use polymers to build a synthetic version of biological tissue plugs. That led us to the design of these off-the-shelf synthetic plugs.”
CC-ROPs consist of two parts: a cartilage cap made of an ultra-strong hydrogel that uniquely mimics the mechanical properties of biological cartilage and an osseous (or bone) base made of a porous, bioresorbable polymer. After implantation, the base is replaced by new bone tissue and continues to anchor the cartilage cap. The cylindrical implant stimulates the formation of new bone tissue and integration with host tissue while supplying synthetic cartilage that’s necessary for joint function.
When designing the plugs, Dr. Grunlan’s team leveraged two unique materials developed in their lab: a hydrated gel that mimics the properties of cartilage and a robust porous foam that is replaced by new bone tissue.
“We merged these two materials into a cylindrical plug, comprising a cartilage cap and a bone-promoting anchor,” she explained. “Importantly, this design provides the potential for ease of translation into clinical practice. They are easily implanted, their size can be tailored and they do not rely on biologics.”
Current options that are used to treat OCDs have their limitations. Autografting involves harvesting small cylindrical specimens from the undamaged part of the patient’s knee and transplanting them into pre-drilled holes in the defect area. However, the usefulness of this method can be limited by the patient’s age — it’s less effective in individuals older than 40 years — and the size of the defect.
Although a total knee replacement is sometimes a patient’s only treatment option, Dr. Grunlan noted that the extensive surgery can lead to postoperative complications.
CC-ROPs are not restricted by age or the size of the defect and offer several other benefits, according to Dr. Grunlan. They match the geometry of cylindrical autografts so they can be implanted with existing surgical tools and techniques. They also don’t require pre-loading with cells or growth factors to induce healing, instead leveraging the unique features of the cap and scaffold base. After implantation, CC-ROPs provide immediate support for joint function, including knee articulation.
Exploring Next Steps
Dr. Grunlan’s team has conducted extensive benchtop testing of the materials that make up the plug and are very pleased with the properties. They are now focused on assessing the plug in preclinical models before advancing to human subjects. The new funding received from the NIH will be critical to this next step.
Dr. Grunlan’s team has a patent portfolio centered on this technology. “Coupling that with our extensive and ongoing research has us well-positioned to move the plug down a commercialization path,” she said. “We hope to establish active industrial partnerships to advance it to the market for human and veterinary patients.”
Dr. Grunlan said her team is currently focused on maximizing the potential of the CC-ROPs to heal osteochondral defects and working on a commercialization plan.
“Overall, our product design represents a feasible approach for clinical translation,” she added.
DC
Dan Cook is a Senior Editor at ORTHOWORLD. He develops content focused on important industry trends, top thought leaders and innovative technologies.




