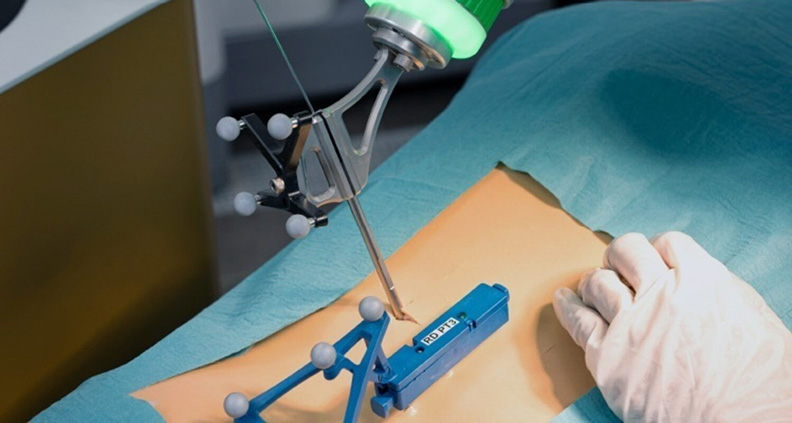
eCential Robotics received FDA 510(k) clearance for a Spine Navigation and Robotic Assistance Device for planning and instrumenting spinal fusion procedures.
The robot was developed in collaboration with DePuy Synthes, which will leverage its reach to bring this solution to market.
eCential Robotics will continue to focus on developing the commercial activities of its Open eCential Platform in the U.S. and partnering with implant manufacturers and tech companies to expand the range of applications available on the platform.
“This additional FDA clearance is a testament to our relentless pursuit of innovation and excellence in the field of surgical robotics and navigation,” said Clément Vidal, CEO at eCential Robotics. “We look forward to continuing to deliver cutting-edge solutions that enhance surgical precision and patient outcomes.”
Source: eCential Robotics
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




