Advancements in 3D printing technologies and build strategies have made it possible for medical device manufacturers to accelerate innovation—from concept to commercialization. Additive manufacturing (AM) has become more cost-competitive with traditional manufacturing thanks to new, more efficient 3D printers, expanded options for materials, proven validation processes, software with deep learning algorithms, regulatory master files and more. Tipping the scale in AM’s favor is the ability to design more complex devices, integrate lattice structures to support bone in-growth, customize and even personalize devices and scale production up and down in response to ebbs and flows in demand.
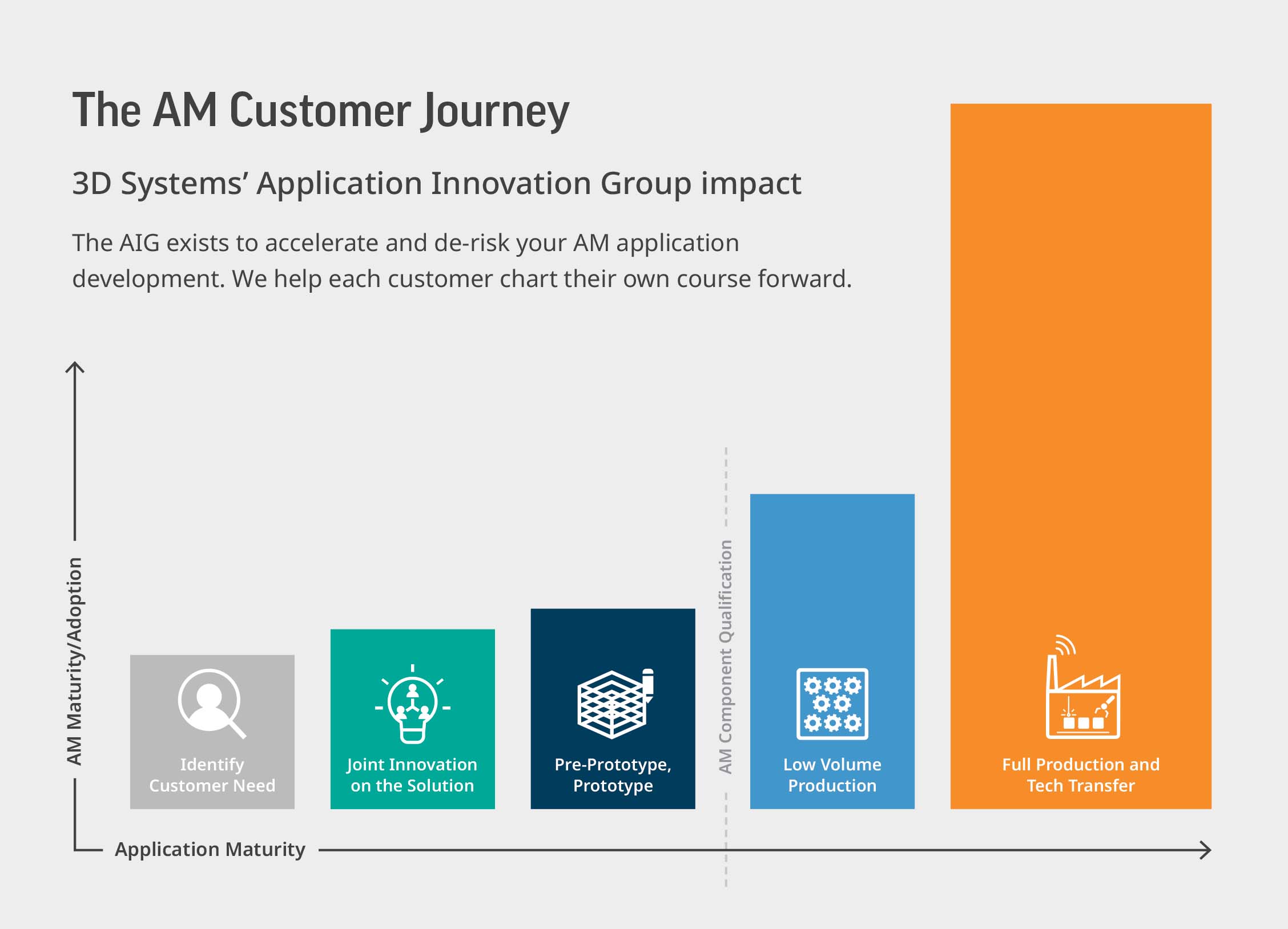
When it comes to expediting device development and regulatory clearance, two major factors have demonstrated the biggest impact: customer-focused experts and a well-defined, well-tested workflow. Leveraging the experience gained over two decades, engineers in 3D Systems’ Application Innovation Group identified the steps they use to approach new customers and healthcare innovations. At each stage, the focus is on understanding the device manufacturer, goals for the new implant or instrument, the end-users’ challenges and devising the right mix of technology, material and design to help meet customer needs.
While 3D Systems has a well-defined model for its approach to healthcare innovation, each new project and customer brings unique challenges. By having a team of experts in-house, we can collaborate and solve issues quickly to expedite the path to submission and beyond.
Let’s explore the steps in this journey:
1. Identify the Need
An in-depth understanding of the application is critical to product development efficiency and ultimately a successful launch. We spend time upfront conducting a thorough screening and analysis to identify a business case for additive manufacturing and formulate a plan for design and development.
2. Joint Innovation
In this stage, we customize our processes according to the objectives defined for the device. Then, we work to choose the right technology and material for the application and collaborate on an optimized design. 3D models are finalized and plans for build optimization are drafted.
3. Pre-prototype/Prototype
Next, we perform a gap assessment against our validated production protocols and modify the new application accordingly. Initial parts are printed and evaluated by customers to confirm that the prototype has the right look and feel and meets their requirements.
4. Low-volume Production
During this stage, we test the device against a series of pre-defined validation and qualification criteria to ensure we have the data needed for regulatory submission and to fuel market adoption once clearance is received. It is at this point that we leverage 3D Systems’ master files to streamline the submission data needed for the new device. We also consult on the best pathway to scale production and leverage our strong partner network to provide recommendations for outsourcing as needed.
5. Full Production and Tech Transfer
Device manufacturers can introduce new products to the market quickly without requiring a costly manufacturing line setup by leveraging manufacturing services from our ISO 13485 facilities. They can also use several medical device contract manufacturers that we’ve enabled with our technologies to ensure a robust supply chain. 3D Systems supports the technology transfer of device manufacturing to customers that wish to build devices in-house. This type of flexibility allows device manufacturers to follow the path that makes the most sense for them, and helps to accelerate, yet also de-risk, the investment in additive manufacturing solutions.
Move from Innovation to FDA Submission in as Little as 90 Days
The typical timeframe for engineering teams to move their orthopedic device innovations from market assessment and prototyping to documentation and submission is 12 months to two years. The industry-accepted average is 20 months. Yet, each month of delay increases the risks of escalating costs and shifting requirements in today’s rapidly evolving medical device industry. Partnership is the key to significantly shortening ideation and validation timeframes and expediting time to revenue. The team at 3D Systems partners with our customers and applies our proven technologies and processes with our expertise in healthcare applications to help make the path to market as quick and easy as possible.
Visit 3dsystems.com/medical-devices to discover more about 3D Systems. You can also schedule a conversation with one of our team members to find out if our approach is right for your next project.
By Ross Attardo, an Application Development Engineering Manager in the Application Innovation Group at 3D Systems.
With nearly a decade as an engineer in 3D printing for healthcare, Attardo is now an expert consultant in managing product realization with 3D Systems’ Application Innovation Group. Leveraging a wealth of knowledge through his previous roles at Johnson & Johnson, Attardo leads design, development and production projects for diverse medical device applications, helping to ensure excellent quality and expeditious delivery. He is a Six Sigma Black Belt and holds a Bachelor of Science degree in Biomedical Engineering from Boston University.