-
Phillips Precision Medicraft
7 Paul Kohner Pl
50 Bushes Ln
Elmwood Park, NJ
07407
United States - 85,000 sq. ft. , 3 Facilities
- 201-797-8820
- 200++ employees
- Founded in 1969
- Operating in North America
- Visit Website
President, Implants and Instrumentation Division
President, Delivery Systems Division
Changes saved

- Cases & Trays
- Instruments
- Logistics/Supply Chain
- Manufacturing
- Materials/Metals
- Product Development
- Regulatory/Quality Assurance
- Surface Preparation/Treatments
Phillips Precision Medicraft is a leading manufacturer of advanced orthopedic implants, instrumentation, sterilization delivery systems, cases, and trays. Founded 56 years ago, PPM has built its reputation on the ability to create innovative and unique solutions that enable OEMs to achieve their most complex goals. Our legacy of precision manufacturing excellence made in the USA, has established PPM as the trusted partner of choice for the world’s leading medical device manufacturers.
For decades, PPM has pioneered industry-defining innovations, building lasting partnerships that turn complex challenges into achievable solutions. Driven by excellence in American manufacturing and a steadfast commitment to quality, we have established ourselves as the premier partner for the world’s leading OEMs.
A History of Innovation
For decades, PPM has pioneered industry-defining innovations, building lasting partnerships that turn complex challenges into achievable solutions. Driven by excellence in American manufacturing and a steadfast commitment to quality, we have established ourselves as the premier partner for the world’s leading OEMs.
PPM has a critical understanding of how to bridge the gap between engineering and manufacturing. We have the benefit of producing thousands of products across a broad range of orthopedic product lines every day. This experience means we provide an unequaled ability to manufacture the highest quality products with the most streamlined supply chain logistics in the business. Every new or repeat product benefits from our integrated DFM (Design for Manufacturing), Lean Engineering and Lean Manufacturing processes.
We maintain a culture of continuous improvement with LEAN Manufacturing disciplines. By fully integrating continuous improvement efforts with value stream partnerships, we are able to adapt our business to fit our customer’s needs exactly.
Bridging the Gap Between Engineering and Manufacturing
PPM has a critical understanding of how to bridge the gap between engineering and manufacturing. We have the benefit of producing thousands of products across a broad range of orthopedic product lines every day. This experience means we provide an unequaled ability to manufacture the highest quality products with the most streamlined supply chain logistics in the business. Every new or repeat product benefits from our integrated DFM (Design for Manufacturing), Lean Engineering and Lean Manufacturing processes.
We maintain a culture of continuous improvement with LEAN Manufacturing disciplines. By fully integrating continuous improvement efforts with value stream partnerships, we are able to adapt our business to fit our customer’s needs exactly.
Phillips operates from three locations in the U.S., combining advanced automation technology with a strong commitment to our people, quality, and global OEM partnerships. Our manufacturing processes are precision-engineered to provide exceptional speed and accuracy while adhering to strict regulatory requirements and meeting aggressive time-to-market demands. By thoroughly implementing LEAN and 5S manufacturing principles along with advanced automation technology, we ensure the quality of our products while systematically increasing our production capacity.
What sets Phillips apart is the hands-on machining experience of our engineering team. This practical knowledge, combined with our deep understanding of OEM needs and requirements, allows us to act as a true extension of our partners’ business. The result is more than just manufacturing; it is a collaborative partnership that consistently delivers scalable and repeatable solutions, driving real success for the OEMs we serve.
PPI: Leading the Way in High-Volume Implant Manufacturing
Phillips operates from three locations in the U.S., combining advanced automation technology with a strong commitment to our people, quality, and global OEM partnerships. Our manufacturing processes are precision-engineered to provide exceptional speed and accuracy while adhering to strict regulatory requirements and meeting aggressive time-to-market demands. By thoroughly implementing LEAN and 5S manufacturing principles along with advanced automation technology, we ensure the quality of our products while systematically increasing our production capacity.
What sets Phillips apart is the hands-on machining experience of our engineering team. This practical knowledge, combined with our deep understanding of OEM needs and requirements, allows us to act as a true extension of our partners’ business. The result is more than just manufacturing; it is a collaborative partnership that consistently delivers scalable and repeatable solutions, driving real success for the OEMs we serve.
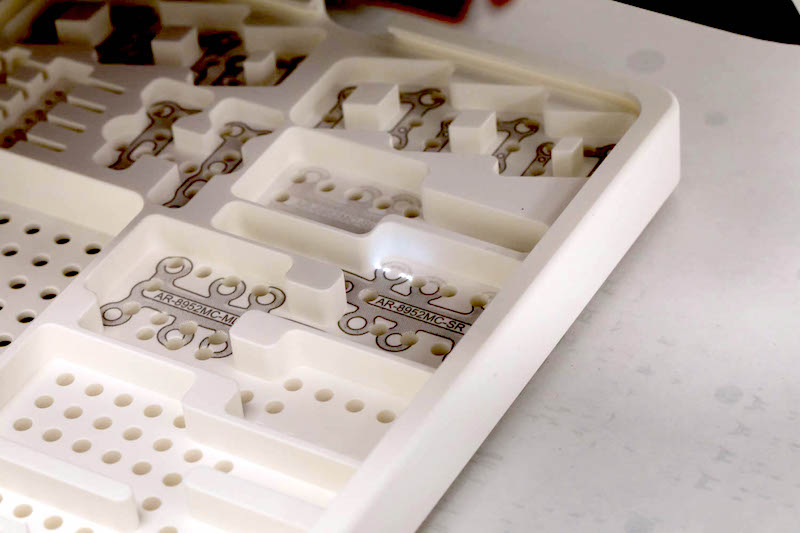
At our Delivery Systems facility, we transform medical packaging into a perfect synthesis of form and function. Our engineering teams don’t just create cases and trays—they orchestrate solutions where every detail serves a purpose. By combining artistic vision with technical precision, we deliver packaging systems that excel in both performance and presentation.
Our innovative DCG™ technology represents this philosophy in action. This proprietary system produces exceptionally vivid graphics that can also be applied on rounded corners. More than just aesthetically superior, DCG™ offers enhanced durability and superior legibility for UDI applications, all while maintaining cost-effectiveness through our in-house production capabilities.
What sets us apart is our comprehensive approach to excellence:
- Engineering expertise that optimizes space, weight, and functionality
- Strict adherence to regulatory requirements and quality standards
- Advanced manufacturing capabilities that ensure consistent precision
- Creative solutions that enhance operating room efficiency
- Cost-effective designs that don’t compromise on quality
In the demanding global orthopedic medical device market, our success is built on three foundational elements: cutting-edge technology, exceptional talent, and long lasting client partnerships.
Where Precision Meets Artistry: The Medicraft Advantage
At our Delivery Systems facility, we transform medical packaging into a perfect synthesis of form and function. Our engineering teams don’t just create cases and trays—they orchestrate solutions where every detail serves a purpose. By combining artistic vision with technical precision, we deliver packaging systems that excel in both performance and presentation.
Our innovative DCG™ technology represents this philosophy in action. This proprietary system produces exceptionally vivid graphics that can also be applied on rounded corners. More than just aesthetically superior, DCG™ offers enhanced durability and superior legibility for UDI applications, all while maintaining cost-effectiveness through our in-house production capabilities.
What sets us apart is our comprehensive approach to excellence:
- Engineering expertise that optimizes space, weight, and functionality
- Strict adherence to regulatory requirements and quality standards
- Advanced manufacturing capabilities that ensure consistent precision
- Creative solutions that enhance operating room efficiency
- Cost-effective designs that don’t compromise on quality
In the demanding global orthopedic medical device market, our success is built on three foundational elements: cutting-edge technology, exceptional talent, and long lasting client partnerships.
We consistently engage and challenge our customers, suppliers, and employees to improve the quality of our products and services while complying with regulatory standards and expectations. Our commitment to steady growth in personnel, education, and technology enables us to successfully manage our OEM customers’ complex product and regulatory expectations.
Because every customer is unique in its operational structure and quality expectations, PPM has developed a world-class DFM process that engages our customers’ engineering and quality departments throughout the DFM and product transfer cycle. We offer a host of services, which include but are not limited to – Dynamic Control Plans, PFMEA Data, First Article Inspection, Device History Records, Process Validation, and Statistical Process Control.
Our standards, OEM partnerships, and overall approach to quality assurance allow us to offer great value to our customers. PPM is proud to be the manufacturing partner of choice to the world’s leading orthopedic OEMs
Meeting Standards & Exceeding Expectations
We consistently engage and challenge our customers, suppliers, and employees to improve the quality of our products and services while complying with regulatory standards and expectations. Our commitment to steady growth in personnel, education, and technology enables us to successfully manage our OEM customers’ complex product and regulatory expectations.
Because every customer is unique in its operational structure and quality expectations, PPM has developed a world-class DFM process that engages our customers’ engineering and quality departments throughout the DFM and product transfer cycle. We offer a host of services, which include but are not limited to – Dynamic Control Plans, PFMEA Data, First Article Inspection, Device History Records, Process Validation, and Statistical Process Control.
Our standards, OEM partnerships, and overall approach to quality assurance allow us to offer great value to our customers. PPM is proud to be the manufacturing partner of choice to the world’s leading orthopedic OEMs








Elmwood Park, NJ 07407
United States
Elmwood Park, NJ 07407
United States
Elmwood Park, NJ 07407
United States
