-
ECA Medical Instruments
2193 Anchor Ct
Thousand Oaks, CA
91320
United States - 100,000+ square feet, 4 facilities
- 805-376-2509 x213
- 150++ employees
- Founded in 1979
- Operating in Europe, Global, North America
- Visit Website
VP Sales & Marketing
Manager, Inside Sales & Customer Support
Changes saved

- Instruments
- Manufacturing
- Packaging
- Product Development
- Sterilization
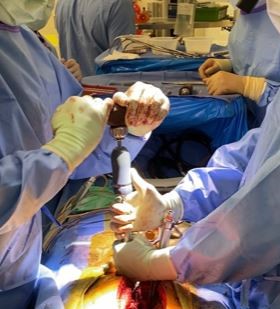
ECA Medical is the #1 choice of orthopaedic implant OEMs and outpatient centers for the design, development & manufacturing of single use sterile pack, surgery-ready procedural kits for trauma, extremities, sports medicine, joint recon and spine. ECA is also a world leader for precision single use torque limiting from 0.5Nm to 14Nm - hand & power operated. With 46 years serving medical device OEMs, ECA has shipped >53 million single-use medical devices and >300K sterile procedural kits
Surgery Ready, Made Easy™. ECA collaborates with OEM implant firms to develop validated sterile pack and surgery ready instrument and implant delivery system solutions for complete range of trauma, sports medicine, upper & lower extremity, large joint and spine implant procedures.
ECA’s surgical solutions team leverages our extensive clinical experience, instrumentation, packaging, validations & process controls to serve as the procedural kit OEM holding DHF and legal manufacturing role to bring sterile pack instruments to market in record time.
The #1 Surgery Ready Choice for Orthopaedic Implant OEMs & Surgery Center Leaders
- Trusted. 46 years of single-use, surgery ready instrument and procedural kit design, development, and manufacturing expertise. An ECA Medical solution is used every 10 seconds of everyday to secure a medical implant.
- Always Ready. Dock-to-stock solutions for trauma, extremities, sports medicine, spine, SI joint, and large joint procedures. Robotic solutions for navigation & implant fixation. ECA partners with all popular implant OEM companies.
- OR Team Benefits. Rapid OR prep, cleanup, and turnover. Eliminate reprocessing costs and staffing issues, curb SSI, and reduce carbon footprint by more than 35%.
- Savings & Efficiency. Optimized case flow with tailored sterile pack and surgery ready instrument sets offering ASCs savings of over $800 per procedure in fixed and variable costs.
- Robust. Employing the latest in materials, ergonomic design & manufacturing technology for instrument and implant kitsConfidence in Hand.
Single Use, Sterile Pack Kits for Orthopaedic Procedures
Surgery Ready, Made Easy™. ECA collaborates with OEM implant firms to develop validated sterile pack and surgery ready instrument and implant delivery system solutions for complete range of trauma, sports medicine, upper & lower extremity, large joint and spine implant procedures.
ECA’s surgical solutions team leverages our extensive clinical experience, instrumentation, packaging, validations & process controls to serve as the procedural kit OEM holding DHF and legal manufacturing role to bring sterile pack instruments to market in record time.
The #1 Surgery Ready Choice for Orthopaedic Implant OEMs & Surgery Center Leaders
- Trusted. 46 years of single-use, surgery ready instrument and procedural kit design, development, and manufacturing expertise. An ECA Medical solution is used every 10 seconds of everyday to secure a medical implant.
- Always Ready. Dock-to-stock solutions for trauma, extremities, sports medicine, spine, SI joint, and large joint procedures. Robotic solutions for navigation & implant fixation. ECA partners with all popular implant OEM companies.
- OR Team Benefits. Rapid OR prep, cleanup, and turnover. Eliminate reprocessing costs and staffing issues, curb SSI, and reduce carbon footprint by more than 35%.
- Savings & Efficiency. Optimized case flow with tailored sterile pack and surgery ready instrument sets offering ASCs savings of over $800 per procedure in fixed and variable costs.
- Robust. Employing the latest in materials, ergonomic design & manufacturing technology for instrument and implant kitsConfidence in Hand.
Confidence on Power. TruPWR torque limiters are designed for use on popular medical power tools. TruPWR reduces surgeon fatigue, enhances OR efficiency and time savings, and improves patient safety with precision fixation of the implant. Fully validated, sterile pack, surgery ready.
Higher torque set points feature 5:1 rpm management to provide precision implant fixation from 5Nm to 14Nm with 1/4 square connectors for virtually all trauma, extremity and spine implant procedures including complete scoliosis cases.
Lower power torque solutons are achieved using ECA’s TruPWR Slim product line with set points from 0.5Nm to 3Nm making them ideal for small bone, trauma, extremity and related cases for precision final fixation – fast and accurate for every procedure & patient.
TruPWR power torque limiters
Confidence on Power. TruPWR torque limiters are designed for use on popular medical power tools. TruPWR reduces surgeon fatigue, enhances OR efficiency and time savings, and improves patient safety with precision fixation of the implant. Fully validated, sterile pack, surgery ready.
Higher torque set points feature 5:1 rpm management to provide precision implant fixation from 5Nm to 14Nm with 1/4 square connectors for virtually all trauma, extremity and spine implant procedures including complete scoliosis cases.
Lower power torque solutons are achieved using ECA’s TruPWR Slim product line with set points from 0.5Nm to 3Nm making them ideal for small bone, trauma, extremity and related cases for precision final fixation – fast and accurate for every procedure & patient.
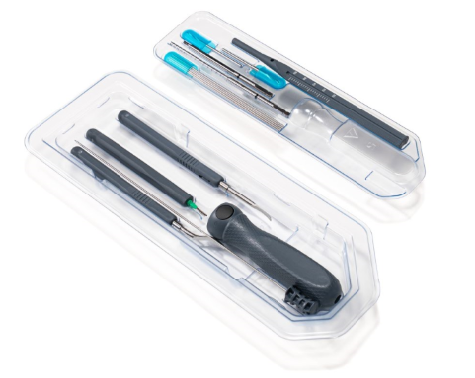
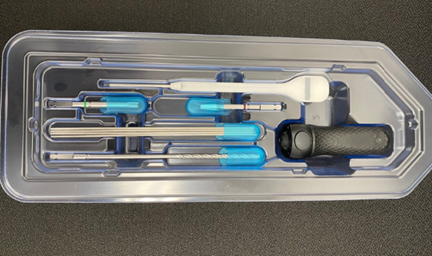
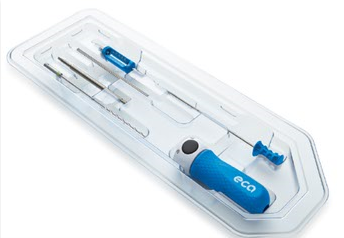
TruTORQ family of single use, sterile & surgery ready precision torque limiters for hand operated fixation of ortho plating, spine pedicle screws and constructs, and joint recon: torque limiting ranges from 0.5Nm to 14Nm with a wide range of handle types including axial, palm, T-handle and Offset T-handle with AO and 1/4 square connectors. These precision factory set torque limiting instruments are off the shelf, fully validated and sterile packed and surgery with labels and IFUs.
TruTORQ Product Line
TruTORQ family of single use, sterile & surgery ready precision torque limiters for hand operated fixation of ortho plating, spine pedicle screws and constructs, and joint recon: torque limiting ranges from 0.5Nm to 14Nm with a wide range of handle types including axial, palm, T-handle and Offset T-handle with AO and 1/4 square connectors. These precision factory set torque limiting instruments are off the shelf, fully validated and sterile packed and surgery with labels and IFUs.
Surgery Ready, Made Easy™. ECA collaborates with trama nd extremity implant OEMs to develop leading edge sterile pack and surgery ready instrument and implant delivery system solutions for complete range of indications for trauma, sports medicine, and upper & lower extremity procedures.
ECA’s surgical solutions team leverages our extensive clinical experience, instrumentation, packaging, validations & process controls to serve as the procedural kit OEM holding DHF and legal manufacturing role to bring sterile pack instruments to market in record time.
Trauma, Extremity & Sports Medicine Procedural Kits
Surgery Ready, Made Easy™. ECA collaborates with trama nd extremity implant OEMs to develop leading edge sterile pack and surgery ready instrument and implant delivery system solutions for complete range of indications for trauma, sports medicine, and upper & lower extremity procedures.
ECA’s surgical solutions team leverages our extensive clinical experience, instrumentation, packaging, validations & process controls to serve as the procedural kit OEM holding DHF and legal manufacturing role to bring sterile pack instruments to market in record time.
Spine & Interventional Pain Solutions. OEM Implant companies partner with ECA Medical for design, development, and manufacturing of optimized and tailored spine and interventional pain management instrument sets for ACDF, facet joint, spinous process, 1 & 2 level lumbar fusion, MIS surgeries, and sacral joint implant (allograft or hardware) prep and fixation.
Spine & Interventional Pain Solutions
Spine & Interventional Pain Solutions. OEM Implant companies partner with ECA Medical for design, development, and manufacturing of optimized and tailored spine and interventional pain management instrument sets for ACDF, facet joint, spinous process, 1 & 2 level lumbar fusion, MIS surgeries, and sacral joint implant (allograft or hardware) prep and fixation.
Joint Recon Solutions. ECA collaborates with implant OEMs to streamline the process flow and add monetized options with a range of surgery ready instruments and sub systems for joint recon procedures. This includes single use, sterile pack and surgery ready components for hip, knee, and shoulder procedures. Expertise includes instrument and complete procedural kits solutons paired with robotics, AI-based systems, imagery and navigation systems and ASC platforms.
Joint Reconstruction
Joint Recon Solutions. ECA collaborates with implant OEMs to streamline the process flow and add monetized options with a range of surgery ready instruments and sub systems for joint recon procedures. This includes single use, sterile pack and surgery ready components for hip, knee, and shoulder procedures. Expertise includes instrument and complete procedural kits solutons paired with robotics, AI-based systems, imagery and navigation systems and ASC platforms.








Thousand Oaks, CA 91320
United States
Thousand Oaks, CA 91320
United States
Draper, UT 84020
United States
