-
Cadence, Inc.
9 Technology Dr.
Staunton, VA
24401
United States - 457,500+ sq. ft., Facilities: 8
- 540-248-2200
- 800+ employees
- Founded in 1985
- Operating in Europe, Global, North America
- Visit Website
Changes saved

- Additive Manufacturing
- Instruments
- Manufacturing
- Materials/Metals
- Packaging
- Product Development
- Surface Preparation/Treatments
- Testing
Cadence is the orthopedic supplier of choice for advanced products used in the orthopedic market. We act as a trusted CDMO partner in developing, manufacturing, and assembling numerous complex products in the areas of spine, trauma, joint replacement and sports medicine. Cadence employs approximately 800 people with its corporate headquarters in Staunton, Virginia and additional locations in Connecticut, Pennsylvania, Rhode Island, Wisconsin, Florida, and Costa Rica.
Cadence collaborates with our customers to solve complex challenges through the entire development process including DFM and DFA. For early-stage products, we have the experience to produce support throughout the different phases of clinical trials as they are an essential part of developing and bringing new products safely to market.
Our Product/Process Development Services Include:
- Product Design Concept
- Design Feasibility
- Design for Manufacturing
- World-class Program Management
- Regulatory & Clinical
- Process Validation
- Manufacturing Transfer
- Product Lifecycle Management
Our expert team, combined with our various services, capabilities, and outstanding customer support, ensure our OEM partners’ product success.
Product/Process Development
Cadence collaborates with our customers to solve complex challenges through the entire development process including DFM and DFA. For early-stage products, we have the experience to produce support throughout the different phases of clinical trials as they are an essential part of developing and bringing new products safely to market.
Our Product/Process Development Services Include:
- Product Design Concept
- Design Feasibility
- Design for Manufacturing
- World-class Program Management
- Regulatory & Clinical
- Process Validation
- Manufacturing Transfer
- Product Lifecycle Management
Our expert team, combined with our various services, capabilities, and outstanding customer support, ensure our OEM partners’ product success.
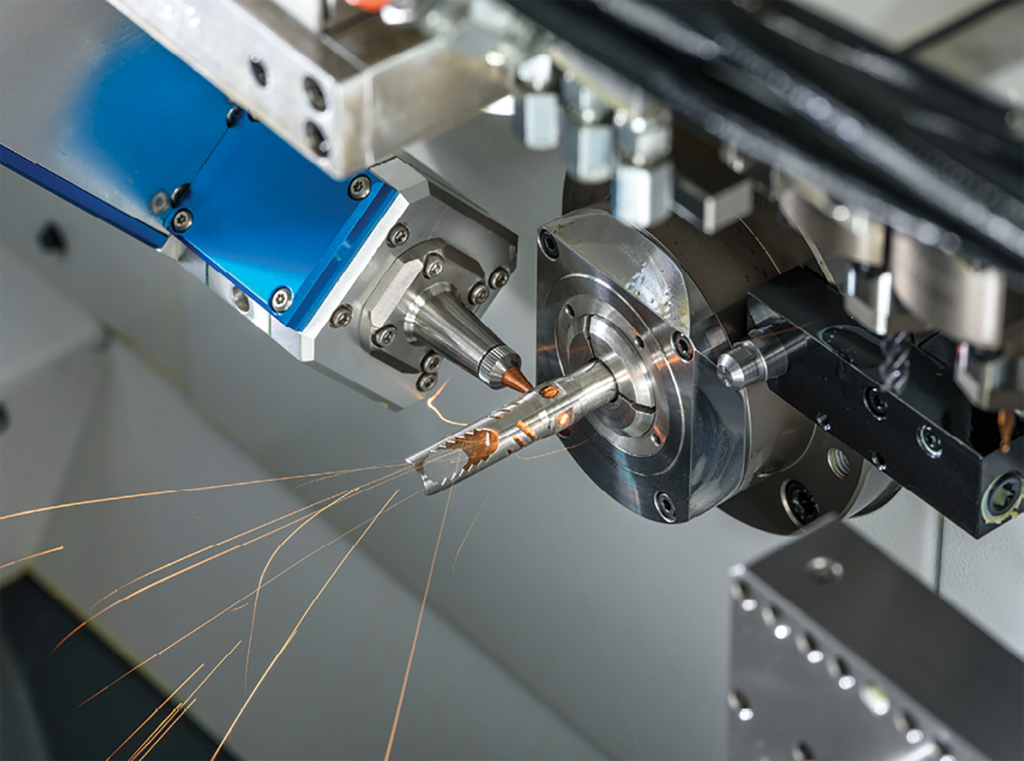
- Sharps – Specialty Blades and Needles
- Machining – Swiss, CNC, Micro, and LaserSwiss
- Laser Cutting, Welding, Drilling, and Marking
- Multi-slide Forming and Microstamping
- Metal Injection Molding (MIM)
- Precision Metal Stamping
- Deep Draw Technologies
- Tube Fabrication
- EDM – Wire, Ram and Sink
- Nitinol Shape Setting
- Advanced Welding Lab™
- IncisionLab™
Metals Expertise
- Sharps – Specialty Blades and Needles
- Machining – Swiss, CNC, Micro, and LaserSwiss
- Laser Cutting, Welding, Drilling, and Marking
- Multi-slide Forming and Microstamping
- Metal Injection Molding (MIM)
- Precision Metal Stamping
- Deep Draw Technologies
- Tube Fabrication
- EDM – Wire, Ram and Sink
- Nitinol Shape Setting
- Advanced Welding Lab™
- IncisionLab™
- 3D Printing
- PEEK Machining
- Insert Molding
- Overmolding
- Cleanroom Molding
- Engineered Resins Expertise
- Sharps Molding & Handling
- Reel-to-Reel Molding
Plastics Expertise
- 3D Printing
- PEEK Machining
- Insert Molding
- Overmolding
- Cleanroom Molding
- Engineered Resins Expertise
- Sharps Molding & Handling
- Reel-to-Reel Molding
Cadence assembles Class I and Class II registered devices for some of the most demanding customers in the MedTech and Pharma markets in our eGMP facilities, including dedicated device facilities in Cranberry Township, PA, and Alajuela, Costa Rica.
Cadence prides itself on a strong track record of regulatory compliance. All of our facilities are ISO 13485 certified and registered with the FDA where applicable. Our robust Quality Management System (QMS) ensures compliance to all regulatory requirements and standards.
We provide the following finished device capabilities:
- Cleanroom Manufacturing (Class 7 and Class 8)
- Final Assembly
- Testing and Inspection Services
- Final Packaging and Labeling
- Sterilization Management
- Supply Chain Management
Finished Medical Devices
Cadence assembles Class I and Class II registered devices for some of the most demanding customers in the MedTech and Pharma markets in our eGMP facilities, including dedicated device facilities in Cranberry Township, PA, and Alajuela, Costa Rica.
Cadence prides itself on a strong track record of regulatory compliance. All of our facilities are ISO 13485 certified and registered with the FDA where applicable. Our robust Quality Management System (QMS) ensures compliance to all regulatory requirements and standards.
We provide the following finished device capabilities:
- Cleanroom Manufacturing (Class 7 and Class 8)
- Final Assembly
- Testing and Inspection Services
- Final Packaging and Labeling
- Sterilization Management
- Supply Chain Management
Staunton 24401
Afghanistan
Cranston 02921
Afghanistan
Suite 400
Cranberry Township 16066
Afghanistan
Sturgeon Bay 54235
Afghanistan
Suffield 06078
Afghanistan
Watertown 06795
Afghanistan
DeLand 32724
Afghanistan
Building B-15
20102
Afghanistan