From regulatory changes to working with new materials and technologies, several aspects of the device testing process can hold up orthopaedic device manufacturers.
Chief among device manufacturers’ frustrations is understanding the technical aspects and specific requirements of testing standards, according to device testing companies.
“Regulatory agencies give OEMs a large series of testing standards that they have to perform and show a successful test. The standards are often just guidelines. They specify the outcome you want and the approximate method for doing a test, but the actual interpretation of the standard, interpretation of the testing method and all of the finer aspects of testing are left up to the company that is performing the testing,” says Trevor Gascoyne, Manager of Clinical Research and Biomedical Engineering, Orthopaedic Innovation Centre. “OEMs say that they need to abide by these standards, show how the testing performed and show their success. At that point, they have a whole slew of questions for us. How many millions of cycles do we do? What kind of loading profiles do we need? For example, FDA requires artificially-aged polyethylene implants. That’s a suggestion of the standard, but not a requirement.”
Keeping an open and strong line of communication between your testing supplier and FDA consultant is critical to meet all of the necessary testing requirements.
Stricter government regulations can also pose problems for device manufacturers when testing their device. Gascoyne notes the increase in requests for aggressive testing for hip and knee simulator or joint wear testing, attributed to recent FDA recalls.
“FDA is proactively combatting this kind of incident for future implants, and what they want to see is the failure mechanism of a particular joint replacement,” Gascoyne says. “In the past, they would test hip and knee implants under ideal conditions in a laboratory, exactly how they were designed to run. What they found with ASR and the metal-on-metal implants was that they weren’t optimally implanted. They weren’t put in the optimal patients under standard ranges of motion.”
Now, according to Gascoyne, FDA is looking for exposure to more adverse, aggressive conditions and asking what happens when an implant is placed incorrectly or fails.
New materials and manufacturing methods may also pose problems for device manufacturers. Challenges with new materials lie in understanding the mechanical performance of the material, as well as which process parameters affect performance, and to what degree, according to Kevin Knight, President, Knight Mechanical Testing.
“On the plastic side, we’ve seen an increase in highly cross-linked polyethylene liners and tibial inserts that have been doped with an oxidation control substance,” Gascoyne says.
“Oxidation, we know from literature and past years, has been very bad for inserts. They start to shear off or delaminate and often crack inside the body, causing a lot of wear very quickly. Some companies come to us with PEEK. This is being applied to similar applications as the standard polyethylene, bearing surfaces primarily. We’ve seen some flexible bearing surfaces. Polyurethane is one of them.”
Knight also notes that additive manufacturing (AM), porous coatings, infused polymers and surface treatments for metals are areas that have experienced rapid advancement.
As newer technologies evolve, so will testing standards.
“With additive manufacturing comes the challenge of understanding the properties of your products and materials, and optimizing them,” says Gemma Budd, Business Manager, Healthcare, Lucideon. “For example, making titanium implants from AM results in an entirely different microstructure to cast or forged titanium, and the resultant impact on mechanical properties—typically weaker—is also clear. Understanding why this is, and how it might be improved, is something on which we are working closely with both the AM companies and the OEMs designing the products.”
“One of our OEMs brought us a 3D printed hip component, and FDA had asked for three or four different aspects of the standard test to be investigated closely,” Gascoyne says. “Part of the fear of 3D printing is porosity inside the implant—the small pores that form during the 3D printing process. Those can be removed using a standard technique of hot isostatic pressing called hipping. Whether or not that removes all of the voids within the implants, we don’t know. Voids can cause fracture and micro motion between the implant, leading to corrosion inside the body.”
Printed metals, produced from powders and sintered via laser or electron beam into a hard object, are another area of concern, Gascoyne says.
“They [FDA] are worried that powder will remain on the surface of these implants and be released into the body slowly or suddenly right after implantation,” he says. “That metal might get into the articulating surface and cause high wear, or get into the modular connections of the implant and cause corrosion. It might cause tissue reactions. As industry is learning more and more about 3D printing and as we’re coming up to pre-product release testing, we’re learning as we go for this kind of system.”

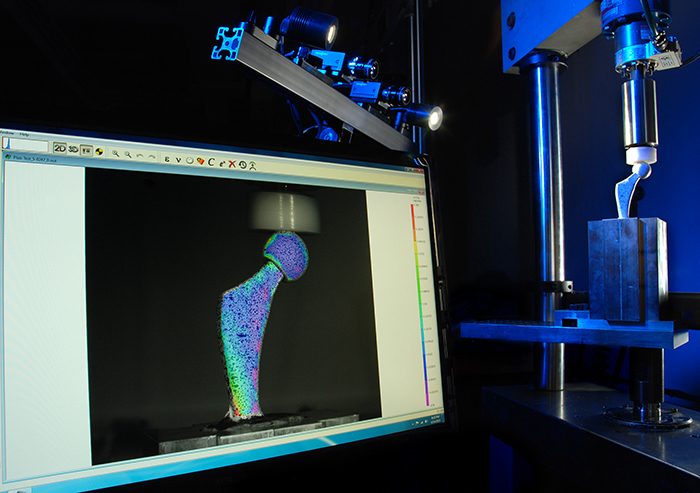
A hip joint under load is analyzed by Digital Image Correlation.
Budd says she sees this with their customers’ products as well.
“They [OEM customers] are relying on our understanding of how materials behave—not just our ability to tell them what the test result is—to help them get to a product that they are confident is safe and effective,” she says.
Testing new devices that have no existing published test methods is another common hurdle.
“Device development typically outpaces standards development, so in many situations we guide our clients in developing custom test methods based on risk analysis, peer reviewed literature and prior experience with regulatory agency demands,” says Knight.
Market segments experiencing growth, such as small total joint replacement, is one example of this, Budd says.
“Aside from the normal mechanical tests, there are some performance properties that do not have standards associated with them,” she says. “For example, wear testing of shoulders, ankles, etc., doesn’t have an ISO or ASTM standard, but with the recent issues with wear debris from hip replacements, manufacturers are unsurprisingly wanting to take a closer look at these attributes, so they have to rely on literature methods and the expertise of their testing partner to design and validate an appropriate method for their products. We are getting asked more and more for this.”
As industry responds to demands from above for innovative, high-quality and low cost devices across the board, this challenge will likely continue.
“Sometimes even where testing standards exist, they are not entirely appropriate for the design of the devices, so they need to deviate from the standard,” Budd says. “Often, finite element analysis (FEA) is used in the earlier stages to help find a design that will give the desired properties, but this is only predictive and is not infallible. When the models do not translate to reality, it is difficult for the manufacturers to understand the failure modes once they have done some destructive testing. That’s why there is a lot of interest at the moment in our Digital Image Correlation service, 3D Strain, which allows you to validate FEA models and visualize in 3D the initiation and propagation of any stresses and strains in real time, before the failure occurs. This information then feeds back into their product design team and is a much more efficient process. This is a quasi-non-destructive testing technique, which helps reduce the costs of testing by requiring [and destroying] less components. Non-destructive testing is another need that is not yet well met in the industry.”
During a presentation at OMTEC® 2015, Brian Choules, Technical Director, MED Institute, offered direction for how device manufacturers should handle a situation where a testing standard doesn’t exist during design verification.
Choules gave five recommendations:
1. Get involved with the appropriate standard committee to publish a new test method. Proposing and leading the development of a new or modified test method can allow your company to collaborate with others in the same situation, and build worldwide acceptance. However, this requires internal resources and time. The typical time frame is two to five years. If the standards community disagrees with the proposed method, write to publications to promote awareness of test methodology; this promotes discussion with data.
Choules says this is the best option, and one benefit is that FDA is usually represented on standards committees. “FDA reviewers can take that information and share it among their colleagues, so that FDA gets a glimpse of best test methods. This can be valuable,” Choules says. “You build synergy when you use a collaborative effort like this. You get more companies following the same method, even before the standard is developed, and that leads to global acceptance.”
2. Validate test method and receive agreement by FDA. You can reduce your level of risk by understanding the uncertainties, range of applicability and comparability to the known method, and increase repeatability and robustness. There is an increased regulatory risk with this tactic. If FDA says that the test method is unacceptable, you must develop a new or modified method and repeat the process.
3. Validate test method and obtain FDA’s formal qualification through Medical Device Development Tools (MDDT) pilot program. MDDT is a way for FDA to qualify tools that medical device sponsors can use when developing and evaluating devices. The program was launched in 2013 to target clinical outcomes assessments, biomarker tests and non-clinical test methods. As a new option, it is not known whether this method will be beneficial for testing in general, as it has the potential to replace industry collaboration and standards.
4. Approach a third party to build equipment. This approach can also be costly. One way to cut down on the cost is to have several companies on a standards committee work together to have equipment built and a new test performed.
5. Form an industry consortium. While also time consuming, this can be useful to obtain otherwise expensive data.
Regardless of the challenges faced with testing requirements, staying flexible and maintaining a strong working relationship with testing suppliers can help to ease the process.
“OEMs have come to us for help when things have changed and we’ve had to adapt very quickly—certainly deadlines need to be met for companies,” Gascoyne says. “It’s difficult to do so when there’s a tight time line and changes occur. Communication is key.”
Send comments on this article to Carolyn LaWell.
Photos courtesy of Orthopaedic Innovation Centre and Lucideon