
I’m pleased to share four companies that received first FDA 510(k) clearances for orthopedic applications within the month of June. These new products reconstruct and fixate bones in various areas of the body, and protect and manage freshly repaired tendons.
Trauma
 Dev4 | Eleganz Fusion Screw System (Fusion Screw System), K250251
Dev4 | Eleganz Fusion Screw System (Fusion Screw System), K250251
Submitted January 2025, granted June 2025
Primary predicate: Acumed Acutrak 3 Headless Compression Screw, K230744
FYI: The system is indicated for bone reconstruction, osteotomy, arthrodesis, joint fusion, fracture repair and fixation of bones appropriate for the size of the device: scaphoid and other carpal fractures, metacarpal and phalangeal fusion and bunionectomies, etc. It is not intended for interference or soft tissue fixation.
Founded in 2024 and located in Houston, Texas, Dev4 is led by a team with over 60 years of experience in orthopedic product development, operations and sales from companies including Exactech, Innovative Medical Device Solutions, Instratek, OsteoMed and Stryker.
 Genostis | Genostis Osteosynthesis System, K242988
Genostis | Genostis Osteosynthesis System, K242988
Submitted September 2024, granted June 2025
Primary predicate: Synthes LCP Low Bend Medial Distal Tibia Plates 3.5mm, K001945
FYI: These plate and screw implants are intended for temporary fixation, correction or stabilization of bones in various anatomic regions (foot, hand, wrist, forearm, clavicle, tibia, humerus, ulna, femur, etc.). The plate/screw interface is locking, non-locking or a combination hole offering both options.
Founded in 2005 and headquartered in Burgdorf, Bern in Switzerland, the company launched the Genostis brand in 2022. They self-describe as a Swiss medical device manufacturer of generic osteosynthesis implant systems, offering the first generic plate and screw system that is MDR certified, 100% made in Switzerland and follows AO Foundation principles of fixation.
Leadership brings experience from roles at DePuy Synthes, Mathys, Medartis, Sulzer, Synthes and Zimmer Biomet.
Sports Medicine
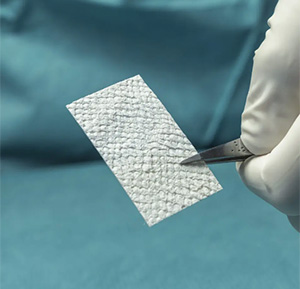 Kerecis | Tendon Protect, K243843
Kerecis | Tendon Protect, K243843
Submitted December 2024, granted June 2025
Primary predicate: Integra Lifesciences Tendon Wrap Tendon Protector, K053655
FYI: Tendon Protect is indicated for the management and protection of tendon injuries in which there has been no substantial loss of tendon tissue.
The product is a solid sheet of intact fish skin intended for the protection and management of newly repaired tendons.
Kerecis is pioneering the use of fish skin and fatty acids in the cellular therapy and regenerative medicine market. The fatty-acid rich (including Omega3) products from the company’s patented technologies enable the body to regenerate tissues, rather than simply repair them and risk scarring.
Headquartered in Isafjordur, Vestfirdir in Iceland and founded in 2011, Kerecis raised $15 million in debt financing in 2022.
 TYBR Health | TYBR Collagen B3 Gel, K250109
TYBR Health | TYBR Collagen B3 Gel, K250109
Submitted January 2025, granted June 2025
Primary predicate: Alafair Biosciences VersaWrap, K213163
FYI: TYBR Collagen Gel is indicated for the management and protection of tendon injuries in which there has been no substantial loss of tendon tissue. The device may also be used in the management and protection of surrounding tissues such as skeletal muscles and ligaments.
B3 GEL comprises a naturally derived extracellular matrix and is applied via TYBR’s proprietary integrated mixer/applicator system. The blue-colored gel allows for visual confirmation of placement and resorbs completely over time without leaving residual bulk.
Preclinical studies have demonstrated that B3 GEL reduced tissue binding and supported improved range of motion in treated models, with enhanced flexion and extension compared to controls.
TYBR Health plans to begin commercial sales by the end of 2025.
TYBR was founded in 2020 and is located in Houston, Texas. The company has raised a total of $2.3 million in funding since 2020, most recently $1.1 million in seed funding at the start of 2025.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




