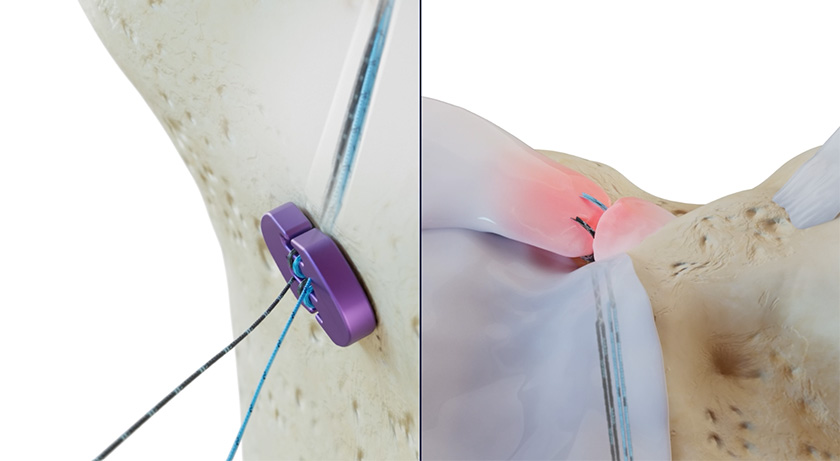
Native Orthopaedics was granted FDA 510(k) clearance to market its Native Root System powered by Dragonfly for meniscal root repair. Limited launch is slated for mid-2Q25.
Dragonfly is a novel advanced quad core knotless finger trap technology that provides instant retensioning capabilities for knee repair procedures. Users can create precise fixation in one motion by simply pulling the end of the device, which tensions and locks, and can be retensioned as desired.
The Native Root System comprises two single-use sterile-packed Dragonfly devices, designed to reapproximate a torn meniscal root back to the native footprint. The Dragonfly platform uses a patented design and manufacturing technique that offers greater mechanical strength compared to basic finger traps and other decades-old technologies.
This “trap within a trap” architecture does not rely on a secondary locking feature to prevent slippage. The suture tension is transmitted directly to the surgeon’s fingertips, allowing improved control and feel during tensioning. Furthermore, the Dragonfly platform is designed to be retensionable, which may allow the surgeon to recover the “unrecoverable” creep.
Testing of the Dragonfly system demonstrated significant advantages over the current standard of care and legacy products. Highlights from the biomechanical testing and simulated clinical use include the following:
- 290% increase in ultimate strength compared to market leader.
- Less than 0.4 mm elongation under cyclic testing.
“With the introduction of the Dragonfly platform to the market, Native Orthopaedics aims to improve the standard of care for all of sports medicine, starting with the meniscal root repair use case,” said Tom Gamache, CEO of Native Orthopaedics.
Source: Native Orthopaedics
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




