
Recent 510(k) clearance recaps have covered enabling technology or focused solely on the shoulder joint. This month, let’s look at new announcements from the segments of sports medicine and trauma. Read on to learn about new solutions for treating bony and soft tissue deformities, fixing wrist fractures and repairing ligament and tendon tears.
Trauma

Orthofix Medical | TrueLok Elevate Transverse Bone Transport (TBT) System, K242861
Submitted September 2024, granted December 2024
Primary predicate: Orthofix TrueLok Hexapod System (TLHEX) V.2.0, K170650
FYI: TrueLok Elevate provides a limb preservation treatment option for addressing bony or soft tissue deformities and defects such as diabetic foot ulcers and nonhealing or deep tissue wounds. While the TBT procedure has previously been reported, TrueLok Elevate is the first dedicated system for TBT to receive FDA clearance.
The product is currently available in a limited release in the U.S. and select international markets.
Orthofix also received approval for the device under the CE Mark, and announced these two regulatory clearances together.

OrthoNovis | BPS Wrist Fracture System, K250498
Submitted February 2025, granted March 2025
FYI: The clearance addresses a line of locking wrist plates designed to fixate certain fractures, fusions or osteotomies in the distal radius.
The company focuses on widely used, market-proven devices that are used to treat common fractures and bone osteotomies. The resulting products incorporate patented and clinically advanced features and are priced significantly less than the competition.
Sports Medicine
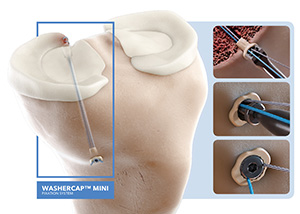
ABANZA | WasherCap Mini Fixation, K250238
Submitted January 2025, granted February 2025
Primary predicate: Abanza Tecnomed WasherCap Fixation System, K212197
FYI: This device is designed for multiple applications, including meniscal root repair and ACL reconstruction.
WasherCap Mini is the first suture and tape fixation device to offer a knotless, bidirectionally tension-adjustable solution, regardless of bone quality.
Biomechanical testing has shown the WasherCap Mini to deliver superior fixation strength and minimal displacement during cyclic loading vs. conventional devices like cortical buttons and suture anchors.
Upcoming developments from the company include the WasherCap In Line and the LoopCap to address procedures like biceps tenodesis, medial collateral ligament repairs and challenging foot and ankle pathologies.

Arcuro Medical | SuperBall-RC System, K244015
Submitted December 2024, granted February 2025
Primary predicate: T.A.G. Medical Products FiberStitch Implant, K221731
FYI: SuperBall-RC is an all-inside, all-suture, knotless meniscal repair device for rotator cuff repair that replaces hard polymer implants with soft, flexible suture bundles. Its tensioning mechanism supports complete control of the repair, mimicking inside-out tensioning of the meniscus.
Proprietary repair suture mesh lies flat, protecting the repaired meniscus and the articulating cartilage, while the SuperBall extracapsular fixation secures the repair and leaves no knots in the joint space.
SuperBall-RC will enter a limited user release in 2Q25 in anticipation of a full launch in 2H25.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




