James Kershner helped develop orthopedic implants at Stryker before shifting his career to focus on human factors related to healthcare packaging designs. He’s now a Director at Bold Insight, where he performs consulting and design work, usability testing and performance tasks related to how easily and effectively medical devices can be used in real-world practice.
Although incorporating human factors principles into packaging designs is crucial, Kershner believes it is often overlooked by orthopedic manufacturers because leveraging pre-existing packaging systems to house new devices is far more appealing than re-engineering a new one with human factors in mind. That logic is understandable.
“Why reinvent the wheel?” Kershner asked. “If the existing solution works, has been validated and used for years without issue, why invest millions to make design changes? There may not be a clear return on investment.”
However, this mindset can lead to missed opportunities to improve the ways devices are used in surgery and contribute to safer patient care and greater satisfaction among surgeons and surgical team members. Optimizing human factors in packaging designs also differentiates devices from the competition and could directly impact their acceptance and success on the market.
Essential Elements
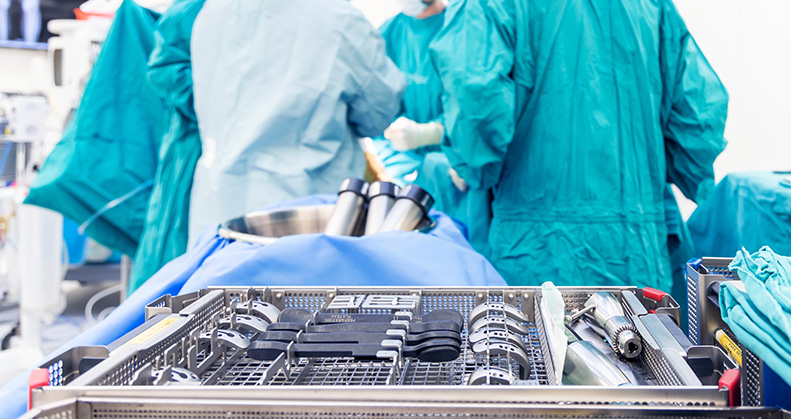
Human factors in orthopedic packaging should be based on three critical aspects, according to Matthew TerBush, Director of Packaging Innovations at Packaging Compliance Labs: the ability to identify the correct way to open the package; recognizing and correctly performing the technique required for aseptic presentation of the device; and properly presenting the package contents to the sterile field.
“Many product engineers might not seriously consider the downstream impact of packaging systems, but the statistics are compelling,” TerBush said.
He noted that 200,000 to 400,000 patients are affected by surgical site infections each year, resulting in $900 million in additional annual healthcare costs, prolonged hospital stays and potentially additional procedures. Smartly designed packaging systems can help mitigate intraoperative infection risks.
From a sustainability perspective, TerBush said, healthcare systems generate over 14,000 tons of waste each day. “By designing packaging that ensures aseptic presentation on the first attempt, we can significantly reduce waste destined for landfills,” he added.
TerBush noted that orthopedic packaging design should help surgical team members maintain sterility, especially in operating rooms where space is limited. “Packaging must minimize the amount of material and contents being transferred to the sterile field,” he said. “Excess layers or unnecessary packaging components can complicate the process and increase contamination risks.”
Packaging designs must account for the personal protective equipment (PPE), such as gloves and gowns with long sleeves, that surgical professionals wear while opening packages to ensure easy manipulation and aseptic transfer.
“By addressing these considerations, packaging systems maintain sterility and support efficient workflows,” TerBush said.
It’s also essential to consider factors like peel strength and other usability aspects when designing packaging solutions. Additionally, multiple devices are often transferred to the sterile field during surgery as a precaution against potential contamination later in the process. It’s, therefore, common to use double sterile barriers for orthopedic devices and implants, according to TerBush.
“This approach ensures that an inner barrier can be transferred to the sterile field and opened when it’s needed to minimize the risk of contamination,” he said.
Outer barrier design considerations differ significantly from inner barrier requirements.
“Outer barriers are typically opened outside the sterile field, which introduces additional challenges and risks,” TerBush said. “First, clear labeling is paramount. The sterile barrier classification should be prominently defined using the latest symbology from ISO standards. This allows surgical team members to quickly identify whether they’re working with a single or double barrier and influences their choice of aseptic transfer technique.”
TerBush noted that ergonomic design is another critical factor for outer barriers and said packaging must be user-friendly to accommodate the needs of PPE-wearing surgical professionals who work under time constraints and in a pressure-packed environment.
“Thoughtful design can enhance usability, minimize errors and improve overall efficiency in maintaining sterility during procedures,” he said.
Transferring devices to the sterile field is fraught with danger. “Issues such as difficulty gripping a tray or awkward handling can lead to contamination,” TerBush said. “To mitigate these risks, it is essential to design packaging that ensures secure handling and usability.”
Multiple access points should be incorporated into the design of a packaging system’s inner barriers, according to TerBush, who noted that features such as finger holes, lift tabs, or similar elements can enhance usability.
“The design should accommodate left- and right-handed personnel, as well as surgical professionals with various hand sizes, to ensure inclusivity and functionality,” he said.
Tear pouches and other packaging barriers can provide additional protection for devices, but their design and usability must be carefully considered. “Since these inner barriers are opened within the sterile field, they allow for different design inputs compared to the outer barrier,” TerBush said. “It’s crucial that opening feature identifiers are clear, bold and prominently marked.”
Additionally, the design of inner barriers must account for the limited space within the sterile field. TerBush said excessive components, such as cushioning, end blocks or large poly bags, introduce unnecessary complexity and increase the risk of contamination. He noted that simplified, protective packaging solutions that balance functionality and efficiency are essential for maintaining sterility and minimizing risks during procedures.
TerBush also recommended that packaging engineers collaborate with surgical professionals. “Their feedback has significantly improved my designs and my perspective as a packaging engineer,” he said. “They’re passionate about packaging — especially when it works well — and they’ll share their enthusiasm for innovative designs.”
Focused From the Start
Kershner suggests bringing in packaging experts to work with product development professionals at the very beginning of the product development cycle. “It’s crucial to address the various ways the new device will impact — or be impacted by — packaging and labeling,” he said. “Most product development professionals are aware that it’s best practice to take this approach, but it’s rarely followed.”
Packaging design teams are often caught off guard when they’re asked to assess human factors when products are ready for validation or formative studies, according to Kershner. It’s understandable that this happens, particularly in large companies, where siloed departments create communication gaps. Still, that doesn’t mean it’s an acceptable practice. Human factors professionals look at products and packaging as a complete unit and need to do that when device designs are being imagined.
“Have them talk through the ramifications and the requirements of a new packaging system before it gets too far down the line and is expensive to address,” Kershner said. “It makes sense to invest in human factors testing then, because the additional cost is relatively small and manageable during product development. It becomes significant after the product is commercialized, when problems are cost prohibitive to fix.”
DC
Dan Cook is a Senior Editor at ORTHOWORLD. He develops content focused on important industry trends, top thought leaders and innovative technologies.




