
It’s always exciting to share the first FDA 510(k) clearances of orthopedic startups. These four players represent the spine, trauma, obio and digital tech segments. They also have a global reach, checking in from Taiwan, Turkey, the U.K. and the U.S. Their solutions improve lateral lumbar spine surgery, enhance the fixation of proximal tibia deformities and spinal compression fractures and help surgeons fine-tune the placement of patella implants during knee replacement surgery.
Spine
 Foundation Surgical | Interwedge Standalone Lateral, K241487
Foundation Surgical | Interwedge Standalone Lateral, K241487
Submitted May 2024, granted October 2024
FYI: The product is used in conjunction with Foundation’s LLIF180 procedure in lateral spinal surgery. LLIF180 is reportedly the first bilateral-fixated and standalone lateral procedure performed through a single-position lateral or oblique-lateral lumbar approach. The LLIF180 procedure’s bilateral fixation is made possible by the Interwedge system’s novel deployable staple. Interwedge also features a hinge mechanism between the spacer and lateral plate which, combined with the deployable staple, provides ipsilateral and contralateral fixation.
The company was surgeon-founded in 2021 and is headquartered in Scottsdale, Arizona. Leadership brings experience from roles at Medacta, Osseus Fusion, Globus Medical and K2M.
Trauma
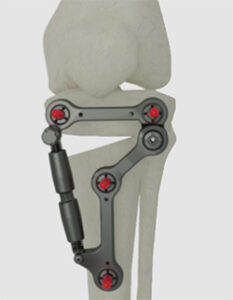 Response Ortho Solutions | Metaphyseal Hinge Fixator, K241769
Response Ortho Solutions | Metaphyseal Hinge Fixator, K241769
Submitted June 2024, granted September 2024
Primary predicate: EBI XFIX DFS Metaphyseal Correction System, K001358
FYI: This unilateral external fixator is designed for the gradual and acute correction of frontal plane, varus and valgus, and metaphyseal deformities of the proximal tibia. The fixator allows up to 20° varus and 10° valgus correction, with the ability to align the hinge to the center of the deformity. The device is reportedly one of the lowest profile external fixators in the market. The anatomical design, 9mm thickness and half-pin locking mechanism support greater patient comfort during recovery.
The company was formed in 2007 with headquarters in the Istanbul Province of Tuzla, Turkey. It focuses on advanced fracture fixation and bone deformity correction with a combination of hardware and Click2Correct preoperative planning and templating software.
Orthobiologics
 Xelite Biomed | XeliteMed VertehighFix High Viscosity Spinal Bone Cement, K241775
Xelite Biomed | XeliteMed VertehighFix High Viscosity Spinal Bone Cement, K241775
Submitted June 2024, granted September 2024
Primary predicate: Tecres Mendec Spine, K042415
FYI: VertehighFix is indicated for fixation of pathological fractures of bone using vertebroplasty and kyphoplasty. Vertebral compression fractures may result from osteoporosis, benign lesions or malignant lesions.
Founded in 2016 and based in New Taipei City, Taiwan, the company markets a variety of bone cements and synthetic bone graft substitutes for spine, orthopedic, dental and craniofacial applications.
Robotic/Digital Technologies
 Eventum Orthopaedics | QUADSENSE, K241298
Eventum Orthopaedics | QUADSENSE, K241298
Submitted May 2024, granted October 2024
FYI: Quadsense provides orthopedic surgeons with a tool for adjusting the patella implant during primary total knee arthroplasty (TKA). It is cleared for any medical condition in which primary TKA is indicated and the patella is resurfaced. It is to be used as a comparative tool for adjusting the patella implant, with a focus on quadriceps force. Quadsense is sterile and designed for single-patient use. As of March 2023, the system was used during 20 robotic surgeries performed in New Zealand.
Eventum Orthopaedics was founded in 2020 and is based in Leeds, Great Britain. The company has raised £3.9 million in funding over two rounds to date, with the most recent a Series A in March 2023. Leadership brings experience from roles at Ethicon, Johnson & Johnson and DePuy Synthes subdivisions as well as JRI Orthopaedics.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




