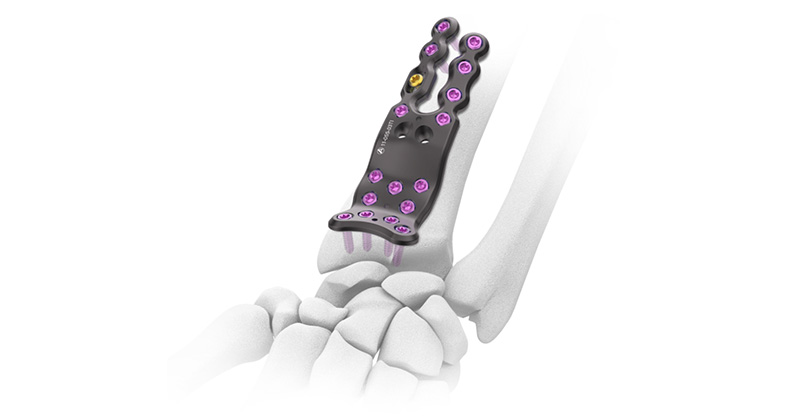
Auxein, a global provider of orthopedic and arthroscopy solutions, successfully achieved European Union Medical Device Regulation (EU-MDR) certification 2017 issued by DNV, Norway. Auxein has made history as the first Asia’s orthopedic implant manufacturing organization to reach this milestone for its Trauma Plating, Screws & Nailing System including Tibia Plate and Screw and Femoral Nailing System.
Auxein offers a portfolio of over 3,000 orthopedic products, designed to meet a variety of patient and clinical needs. All products are CE-certified and fully compliant with the European Medical Device Directive MDD/93/42/EEC, as amended 2007/47/EC. Additionally, a selected range of products has been US FDA 510(k) cleared, further validating the safety and efficacy of Auxein’s product offerings for global markets.
Mr. Gaurav Luthra, Vice President Global Manufacturing and Regulatory Head at Auxein, stated, “Securing EU-MDR certification is a testament to our relentless dedication to providing the safest, most innovative orthopaedic solutions. This achievement, combined with our extensive global certifications, positions us to drive advancements in healthcare on a global scale.”
Source: Auxein Medical
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




