
The run-up to the American Academy of Orthopaedic Surgeons Annual Meeting is full of showcase previews that allow exhibiting companies to highlight products and solutions that will be on display in their booths. The news doesn’t stop breaking when the conference starts. These announcements were shared from the show floor about new product releases, strategic partnerships and regulatory milestones.
Bone Solutions Gains Expanded Indications for Mg OSTEOCRETE
Mg Osteocrete
The company received FDA 510(k) clearance to market Mg OSTEOCRETE bone substitute for use in the intervertebral body disc space, including for cervical, thoracic and lumbar fusion procedures. Mg OSTEOCRETE is the first magnesium-based bone substitute to be cleared by FDA for this application.
The product remodels into bone over time through creeping substitution. This remodeling profile means that the material provides needed support to the defect or void during the healing process, then transforms into bone at an optimized rate. Mg OSTEOCRETE is already cleared for use in posterolateral spine procedures.
Osso VR Debuts New Hand Control Feature

Osso VR Hand Control
Osso VR, a provider of immersive surgical training tools, introduced Hand Control, a controller-free option designed to complement and enhance the training capabilities available to healthcare professionals during virtual reality (VR) training scenarios. This new feature provides a realistic, intuitive range of motion without the need for traditional handheld controllers.
Hand Control leverages Meta’s latest hand-tracking APIs that employ cameras on the VR headset to track hand and finger movements. The technology seamlessly supports all standard controller interactions, giving users the freedom to choose between hands-free gestures and traditional controllers for a more personalized and immersive experience. This latest feature can enhance and better simulate real-life clinical scenarios, providing realistic and effective learning tools for orthopedic residents and surgeons.
Smith+Nephew Introduces AETOS Shoulder
AETOS Shoulder System
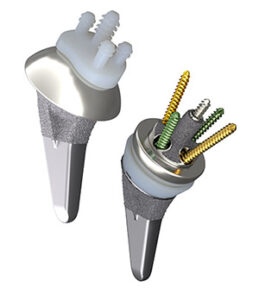
Smith+Nephew commenced full U.S. commercial availability of its AETOS Shoulder System and announced FDA 510(k) clearance for its use with ATLASPLAN 3D Planning Software and Patient Specific Instrumentation for total shoulder arthroplasty.
AETOS Shoulder restores patients’ range of motion and helps to minimize arthritic shoulder pain. The system features the Meta Stem which is designed for stability with metaphyseal fixation and an inlay collar, bone preservation, and to maintain patient anatomy.
Indicated for both anatomic and reverse applications, AETOS Shoulder offers a compact but full portfolio. With fewer steps for conversion and fewer instruments needed to perform primary anatomic and reverse replacements, the system is designed to simplify flow in the O.R. or ASC.
ATLASPLAN 3D Planning Software and Patient Specific Instrumentation supports preoperative case planning with:
- A user-friendly web-based planner for access from any computer or tablet.
- Fast turnaround time from image upload to planning.
- An optional 3D-printed glenoid guide, designed with a patented coracoid clip for stability and offering a reliable method to execute the plan through conventional surgical techniques.
Stryker Debuts Knee Implant and Mako Upgrades

Triathlon Hinge Knee
Stryker introduced the new Triathlon Hinge Knee and highlighted the continued evolution of Mako SmartRobotics.
Triathlon Hinge is designed to reduce the procedural steps of a Triathlon revision-to-Hinge conversion during surgery with streamlined workflow and simplified instrumentation. The system addresses challenges that a surgeon may encounter during a revision and provides surgical workflow efficiency.
Stryker also introduced updates to the Mako surgical robot platform.
With over 56% of surgeons in the U.S. performing hip replacement through the direct anterior (DA) approach, Stryker now offers a solution focused on reducing intraoperative fluoroscopy in DA procedures. Mako SmartRobotics, part of the Direct Anterior Reconstructive Technology (DART) ecosystem, has demonstrated accuracy to surgical plan in total hip arthroplasty without the use of fluoro.
Stryker is also extending a surgeon’s Mako SmartRobotics experience in and beyond the O.R. through the development of the myMako app for Apple Vision Pro and the iPhone. When used on Apple Vision Pro, myMako allows surgeons to visualize and review patients’ Mako surgical plans in an immersive visual experience.
Tetrous Launches EnFix TAC

EnFix TAC
Tetrous added EnFix TAC to its family of EnFix demineralized bone allograft implants for use in rotator cuff surgery.
Tetrous is focused on meeting an unmet need in bone-to-tendon healing. Numerous clinical and preclinical studies show that the enthesis does not re-form following surgery, but instead results in the formation of scar tissue, which does not allow proper transmission of tensile load from soft tissue to bone and is prone to fail.
EnFix TAC is designed to deliver enthesis healing properties while offering the flexibility to be used independently of the surgeon’s suture anchor choice.
While Tetrous’ initial products focus on the rotator cuff, the company is also working on additional applications for ACL and Achilles tendon repair as product line extensions to the EnFix family.
Tyber Medical Gains FDA Clearance for Fracture Plates
Proximal Tibia Plating System
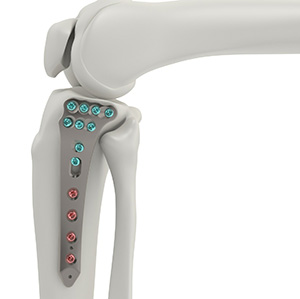
Tyber Medical’s Proximal Tibia Plating System comprises two indication categories: Complete Articular and Partial Articular Fractures. Both designs stabilize bone fragments to facilitate the healing of a wide array of injuries to the tibia, fibula and femur.
The Proximal Tibial Plating System is engineered to accommodate surgeon preferences, increase procedural efficiency and enhance plate fit and screw placement. It is anatomically designed to address a variety of indications and includes variable angle locking and non-locking screws.
THINK Surgical Collaborates to Expand Coverage
An agreement with Waldemar Link will add the LinkSymphoKnee total knee replacement system to THINK Surgical’s ID-HUB (implant data hub), a proprietary databank of implant modules for use with its TMINI Miniature Robotic System. Additional collaborations will add Maxx Orthopedics’ Freedom Knee and b-ONE Ortho’s MOBIO Total Knee to the databank.
THINK Surgical’s databank already includes five implant systems from four other manufacturers. Adding additional implants to the database enhances surgeons’ options when using the TMINI system, which includes a wireless robotic handpiece for use during total knee replacement.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




