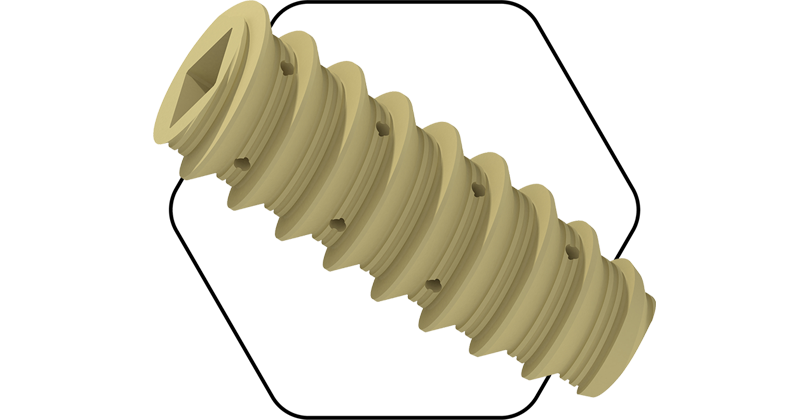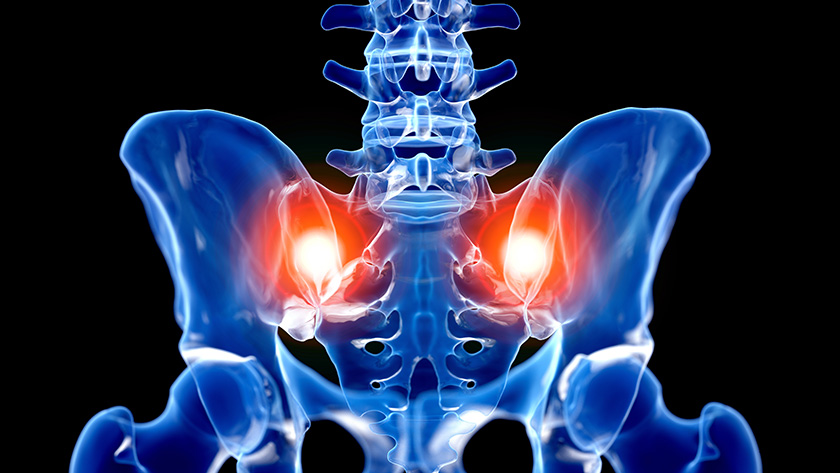
Spinal Simplicity was granted FDA 510(K) clearance to market the Liberty-SI Lateral Implant, a transfixing, lateral sacroiliac (SI) joint fusion system. Liberty-SI Lateral is intended for sacroiliac joint fusion for conditions including sacroiliac disruptions and degenerative sacroiliitis.
The procedure involves lateral insertion of one or two small titanium implants transfixing the SI joint, designed to stabilize and fuse the joint. The lateral surgical technique first decorticates the bone, preparing the joint for fusion. The Liberty-SI implant is designed to achieve arthrodesis of the SI joint using a lateral technique that passes through the ilium across the sacroiliac joint and into the sacrum, thus transfixing the sacroiliac joint.
Soft launch is slated for early 1Q24, with full market release to follow in 2Q24.
Source: Spinal Simplicity
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.