The future of spine is coming into focus. Minimally invasive surgery will advance as orthopedic companies develop low-profile and adaptable devices applied through small incisions. Discs will be replaced with motion-preserving implants instead of fused vertebrae. Novel implant materials will promote bone ingrowth and improve vertebrae stability. Developments in regenerative medicine will create environments that are conducive to cell growth and healing with the goal of delaying surgery or preventing it altogether.
Kevin McGann, President & CEO of Accelus, expects minimally invasive spine care to become more widespread and accessible as cases continue to move to outpatient settings.
“Improvements in surgical techniques and technologies will lead to shorter recovery times, reduced hospital stays and less postoperative pain for patients,” he said. “We also anticipate an increased emphasis on procedures performed at ambulatory surgery centers, allowing more patients to benefit from these less invasive approaches.”
There are a lot of exciting developments in spine — the largest individual segment in orthopedics — that presents opportunities for future growth in the design of low-impact devices that fuse, replace and regenerate diseased or damaged discs.
Achieving “PEEK” Performance
The advancement of new biomaterials is the most significant development in spine surgery, according to Ryan Roeder, Ph.D., Founder and Chief Technology Officer of HAPPE Spine. He pointed to the growing interest in polyetheretherketone (PEEK) devices that incorporate porosity and enhanced tissue integration in spinal implants.
About 20 years ago, Dr. Roeder noted, PEEK implants addressed specific challenges associated with dense titanium devices, which at the time were rigid and didn’t effectively transfer loads to the graft space to promote fusion. The titanium devices were also challenging to image, particularly postoperatively, making it hard to assess fusion success.
“When PEEK implants were first introduced, they facilitated load transfer to the graft space — mechanical loads play a pivotal role in bone formation — and enhanced the osteogenic response,” Dr. Roeder said. “PEEK implants also allowed surgeons to see what was happening within the graft space.”
But traditional PEEK is inert and hydrophobic and can lead to fibrous tissue growth around the implant. “This limitation was manageable until 3D-printed titanium implants with porous structures emerged,” Dr. Roeder noted. “Titanium is known to be osteoconductive — bone can grow along its surface and even into its pore spaces. These factors addressed the previous deficiency of PEEK.”
Dr. Roeder heard the buzz about 3D-printed titanium implants at spine society meetings. “Surgeons were excited about their osteoconductivity, which could result in better stability, improved outcomes and potentially faster fusions,” he recalled. “However, they still struggled with imaging and had concerns about implant rigidity.”
When asked how 3D-printed titanium implants could be improved upon, one surgeon suggested making PEEK implants similarly osteoconductive. Dr. Roeder smiled and told them what HAPPE was working on.
“We’re focused on creating porous and surface-active PEEK devices, which have become a significant area of interest,” he said. “Multiple other companies are also working to bring related products to market.”
Spine has seen multiple transitions in implant materials from dense titanium to dense PEEK to porous titanium, Dr. Roeder noted. “The next logical step is porous PEEK, which combines the advantages of both materials,” he added.
When manufacturing porous PEEK, Dr. Roeder noted, addressing the surface becomes crucial. Some methods, such as coatings or fillers, make the surface of PEEK more hydrophilic and osteoconductive than PEEK alone.
“We incorporate hydroxyapatite into the PEEK material to treat the surface,” Dr. Roeder said. “Other companies may use surface coatings. Our surface modification is a key component of our implant material formula.”

The first clinical case with HAPPE Spine’s INTEGRATE-C Interbody Fusion System took place in August.
HAPPE Spine’s INTEGRATE-C Interbody Fusion System received FDA clearance in May 2023 and was used for the first time in surgery three months later. The fusion cage is said to be the first of its kind to fully incorporate both porosity and hydroxyapatite throughout the entire structure.
Unlike previous PEEK implants that only had porosity on the end plates, INTEGRATE-C’s porosity extends from plate to plate. “This design allows for the comprehensive integration of the graft window, which is crucial for fusion and biomechanical stability,” Dr. Roeder said. “We want the bone to quickly grow through the window and then seamlessly integrate with the implant.”
INTEGRATE-C’s cage is described as radiolucent and radiovisible, which offers the best of both worlds in terms of imaging performance. “Although we’ve included markers, such as tantalum spheres, to meet FDA requirements, some surgeons have suggested they aren’t necessary because the implant looks like bone right from the start thanks to the significant presence of hydroxyapatite,” Dr. Roeder said.
INTEGRATE-C also addresses the issue of fibrous tissue encapsulation. Surgeons favor the idea of having a PEEK implant that can fully integrate with the surrounding bone during fusion, Dr. Roeder notes. “Our INTEGRATE branding plays off the concept of osseointegration to highlight our unique combination of porosity, hydroxyapatite and imaging capabilities into a single implant.”
Novel Designs Promote Personalized Care
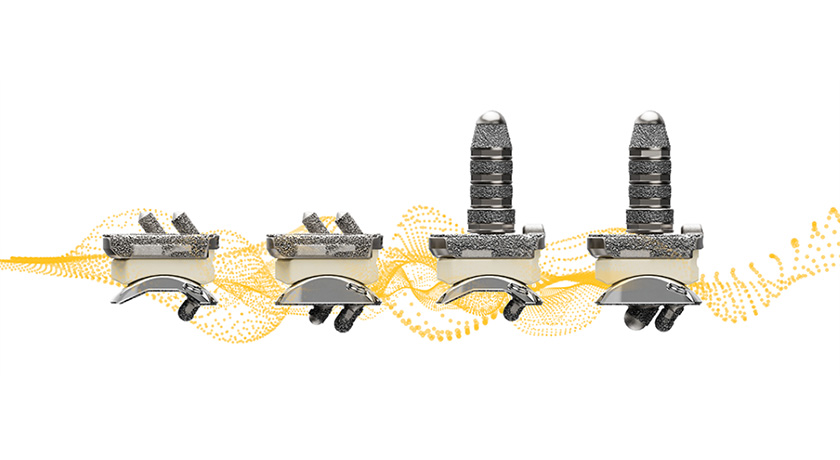
Accelus’ Adaptive Geometry technology advances minimally invasive spine surgery and has been incorporated into the company’s flagship FlareHawk product portfolio and the Toro-L Interbody Fusion System. McGann said the technology allows for a minimal insertion profile during surgery, easing the challenges of accessing and supporting the interbody space. This ensures a maximum interbody footprint and minimizes the risk of subsidence, a common concern in spinal fusion procedures.
The Adaptive Geometry technology optimizes the implant’s shape to fit a patient’s anatomy precisely and enhances load-sharing capabilities, factors that increase the probability of successful fusion. Additionally, the minimal insertion profile reduces tissue disruption and the need for extensive retraction, contributing to faster recoveries and reduced postoperative pain.
The FlareHawk devices’ multidirectional interbody expansion capability also plays a key role in meeting unmet clinical needs. By expanding in multiple directions, the device restores foraminal height, reduces the risk of subsidence and reestablishes sagittal balance.
“These outcomes are crucial for patients with complex spinal pathologies, as they contribute to greater spinal stability and alignment,” McGann said. “FlareHawk’s open architecture also facilitates maximum bone graft delivery into the disc space, which leads to robust fusion.”
The device’s adaptability optimizes graft placement and further contributes to successful bone fusion. Its endplate design allows the cage to conform precisely to each patient’s unique topography. “This personalized fit increases the bone-implant interface’s surface area, enhancing osseointegration and improving load distribution across the endplate,” McGann said.
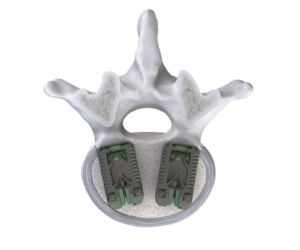
TiHawk11 is part of Accelus’s flagship FlareHawk Interbody Fusion System.
Accelus’ TiHawk portfolio of expandable interbody cages features a titanium surface technology that preserves the favorable properties of PEEK while also offering the enhanced bone-growth properties of titanium. Unlike traditional all-titanium implants, TiHawk does not generate a ghost artifact under fluoroscopic imaging. This allows for easy radiographic visualization during implant placement and fusion assessment.
The newest line of TiHawk implants (TiHawk11) gained FDA clearance in May 2022. The line features an 11mm-wide insertion profile that expands to 17mm in width and up to 14mm in height within the disc space. This significant expansion provides surgeons with a 70% increase in footprint space compared to a standard 10mm-wide interbody device. Additionally, the expandable design of TiHawk11 enables the insertion of two 17mm-wide implants via a posterior approach, effectively creating a 34mm-wide footprint within the disc space.
TiHawk11’s expanded footprint, bone-growth properties and ease of radiographic visualization are examples of innovation that help surgeons achieve stable fusion and improved outcomes.
High-tech Care Contributes to the Outpatient Migration
The field of enabling technology for spine surgery is continuously evolving with research and product development efforts focused on enhanced surgical navigation, robotic-assisted surgery and advanced imaging capabilities. Accelus recently sold its Remi Robotic Navigation System to ATEC, but McGann said that enabling technology will continue to play a role in the delivery of spine care.
“We anticipate that these platforms will become increasingly integrated with each other and surgeon and hospital requirements, allowing for seamless and more precise surgical interventions in the coming years,” McGann said.
He believes artificial intelligence (AI) and machine learning have the potential to revolutionize spine surgery by providing valuable insights into patient outcomes and the effectiveness of personalized surgical plans and devices.
“By analyzing vast amounts of patient data, AI can assist surgeons in making more informed decisions and tailoring treatments to individual patients,” he said. “This will contribute to better patient outcomes and the delivery of value-based care, ensuring that treatments are effective and cost-efficient.”
McGann noted that the continued movement of cases to ASCs has increased interest in enabling technology. It has also increased the importance of disposable or streamlined instrumentation sets, which improve surgical efficiencies and lessen the burden on sterile processing departments.
“Orthopedic companies are being challenged to optimize their manufacturing processes to accommodate ASC requirements and must invest in R&D efforts to create efficient, procedure-specific, single-use instrument and implant sets,” he said. “Companies must be proactive and strategic in their approach to meet this growing demand.”
Growing Interest in Motion Preservation
Artificial disc replacement is the future of minimally invasive surgical care, according to Ali H. Mesiwala, M.D., a fellowship trained surgeon in complex spine surgery and a partner at the renowned DISC Sports & Spine Center in Newport Beach, California. He performs about 200 disc replacements annually with the latest in minimally invasive surgical techniques.
“One of the fundamental principles of spine surgery is to reestablish or preserve normal anatomy and function,” Dr. Mesiwala said. “Disc replacement within spinal surgery is a recent area of interest for industry. Cervical arthroplasty is becoming more common, but lumbar arthroplasty is gaining in popularity.”
Motion preservation re-establishes the spine’s natural mobility and, in most cases, prevents disease in adjacent vertebrae levels caused by the fusion of spine segments.
“The designs of artificial discs have improved over the last two decades — the biomechanics are closer to the spine’s normal function,” Dr. Mesiwala said. “The surgical techniques used to implant these devices have also improved. The goal is to remove the entire diseased disc and replace it with a prosthetic in the least invasive way possible.”
Newer implants provide a combination of disc replacement and facet joint replacement technologies. In August, ZimVie gained FDA premarket approval for a smaller height of the Mobi-C Cervical Disc portfolio. Mobi-C is the first cervical disc implant that is FDA approved for the reconstruction of a cervical disc at one and two levels. Surgeons insert the cobalt chrome alloy and polyethylene mobile-bearing prosthesis in a single step, without the need to chisel bone or anchor it in vertebrae with screws or keels.
“Surgeons have more options to tackle a variety of challenging anatomy and deformities,” Dr. Mesiwala said. “It’s possible to correct a complex deformity — such as thoracolumbar scoliosis — with a motion-preserving surgery that reestablishes the spine’s normal mobility.”
Sustainable and Regenerative Outcomes
Frank Yohe, Global Director of Natural Materials at DSM Biomedical, said innovation in spine has centered around the refinement of surgical techniques, incremental changes made to dilation systems and the fine-tuning of rods, screws and instrumentation to accommodate smaller surgical approaches. He believes limiting the focus of innovation to device design improvements would miss opportunities for more significant advancement.
“It’s essential to expand our options, which can provide greater freedom in designing surgical procedures,” he explained. “We are currently witnessing a growing interest in materials science, pushing the boundaries of what materials can achieve and building a deeper scientific understanding of them. This shift toward exploring biomaterials is where the next phase of innovation will emerge.”
Last year, DSM Biomedical entered into a supply and commercialization agreement with Dutch-based startup NC Biomatrix, which developed VitaDisc, a breakthrough treatment for intervertebral disc degeneration (IVDD) that triggers the native regenerative mechanisms of cartilage tissues. In February, NC Biomatrix received a €2.5 million grant from the European Innovation Council (EIC) to complete preclinical trials of VitaDisc and run its first in-human trial.
VitaDisc addresses the limitations often associated with back pain and related issues, which typically involve mechanical pressure and biological deficits that lead to pain and nerve root impingement. Traditional injections often lack the ability to effectively address the mechanical pressure on the nerve root.
“Early outcomes data suggests that VitaDisc reduces the mechanical impact on the nerve root and positively influences the biological healing process,” Yohe said. “The double impact occurs simultaneously. This aspect is extremely exciting.”
Stopping the cascade of ailments caused by IVDD has the potential to prevent further degradation — such as annular tears or disc herniation — and promote proper disc regeneration.
Regenerative medicine is one of the most sustainable approaches to patient care, according to Yohe. “It involves harnessing the body’s innate ability to heal itself without introducing unnatural elements,” he said. “Reducing the need for traditional surgery contributes to improved patient care at a lower cost, meeting the essential criteria for sustainable growth.”
VitaDisc is a notochordal cell-based injectable harvested from the spines of pigs and manufactured with an environmentally friendly production process in which 100% of the manufacturing is green and environmentally responsible.
“This approach checks all the boxes in terms of environmental responsibility, healthcare efficacy and economic viability,” Yohe said. “It’s an example of how we can improve patient care and contribute to a more sustainable world.”
DC
Dan Cook is a Senior Editor at ORTHOWORLD. He develops content focused on important industry trends, top thought leaders and innovative technologies.