
Joint Replacement
Total Joint Orthopedics | Klassic Knee Revision System, K230537
Submitted February 2023, granted May 2023
Primary predicate: TJO Klassic Knee System, K112906, K140942
FYI: The clearance includes a femoral cemented stem and designs of femoral augments, tibial augments and revision cones.
The femoral cemented stem represents TJO’s first step in developing a knee revision system with streamlined instrumentation specifically designed for the outpatient setting.
A standard cruciate retaining primary knee can be converted to a constrained, stemmed construct with just one additional instrument tray and minimal inventory on hand, offering suitability for use in an ASC.
The first case performed with the Klassic Knee Revision System occurred in July 2023.
Trauma

Bone Bolt Fixation
University Of Utah, Department of Orthopaedics | Bone Bolt System, K230867
Submitted March 2023, granted June 2023
Primary predicate: DePuy Synthes Cortex and Cannulated Screws, K161616
FYI: Bone Bolt is a novel implant designed for percutaneous bone fracture fixation. The system comprises implants of various lengths and diameters, along with associated surgical instruments and sterilization trays. The implants are used to treat challenging fractures, such as pelvic fractures and fractures of the long bones in the arm and leg.
Bone Bolt is protected by U.S. Patent No. 11,553,948, with other U.S. and international patents pending. The clearance marks the first time that a novel medical device developed at the University of Utah has received an FDA 510(k) clearance.
The University of Utah’s Orthopaedic Innovation Center (OIC) and PIVOT Center will establish industry partnerships to commercialize the Bone Bolt System and bring it to hospitals and surgery centers throughout the U.S.
Sports Medicine
Anika Therapeutics | Integrity Implant, K223538
Submitted November 2022, granted August 2023
FYI: Integrity was designed to augment an injured tendon to promote healing in rotator cuff repair. Clearance of the hyaluronic acid (HA)-based patch component joins prior 510(k)s received for associated fixation devices and instrumentation.
The Integrity implant is a flexible knitted HA-based scaffold that provides improved strength and regenerative capacity over first-generation collagen patches. It supports regenerative healing through improved cell infiltration, tissue remodeling and tendon thickening.
The implant system comprises the HA-based patch, fixation implants and single-use arthroscopy instruments. The patch component is a porous, flexible construct knitted using HYAFF fibers, and is designed to support cell infiltration and regenerative healing. HYAFF HA technology resorbs over time as surrounding tissue remodels.
A limited U.S. launch is slated for 1Q24, with a full U.S. market release and expansion into international markets to follow.
Robotics/Digital
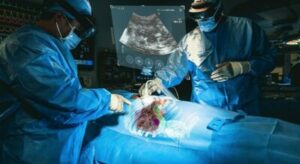
MediView XR XR90 Platform
MediView XR | XR90 Augmented Reality-based Surgical Visualization and Navigation Platform, K223125
Submitted October 2022, granted July 2023
Primary predicate: Real View Imaging HOLOSCOPE-I, K210072
FYI: XR90 is intended for adjunctive use in minimally invasive ultrasound and CT-guided needle-based procedures that treat soft tissue and bone.
This is reportedly the first 510(k) clearance for an AR device that combines live imaging with 3D XR visualization for pre- and intra-operative indications for use.
The device uses AR to precisely overlay highly detailed and colorized 3D CT images of a patient’s internal anatomy — including organs, bones and vasculature— along with real-time tracked locations of instrumentation. The result is an unprecedented view of the patient’s anatomy in full depth and sharp detail.
Through Microsoft’s HoloLens 2 AR headset, clinicians can visualize the patient’s ultrasound images and displays of other procedural information to facilitate their workflow.
Clinicians at remote locations can use XR90 to collaborate in real-time with providers at the bedside through shared visualization and communication.
Recently, MediView XR secured a $15 million strategic funding round with backing from leading collaborators including Mayo Clinic, Cleveland Clinic and GE HealthCare.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




