
These four spinal fusion devices that recently received FDA clearance come from smaller U.S. companies. Among the design features are an element that eliminates the need for bone grafting, a silicon nitride biomaterial, fully integrated porosity and a hydroxyapatite surface treatment designed to enhance healing.
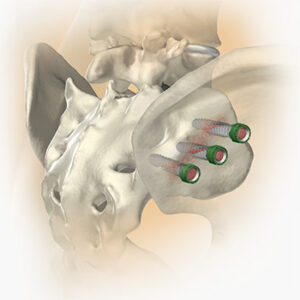
SI-Cure Sacroiliac Joint Fusion System
Alevio Spine | SI-Cure Sacroiliac Joint Fusion System, K223819
Submitted December 2022, granted January 2023
Primary predicate: Frontier Devices FUSIO Screw Fuze System, K141106
FYI: This is the company’s first FDA 510(k) clearance. The SI-Cure Sacroiliac Joint Fusion System consists of titanium bone screws of various diameters and lengths to accommodate patient anatomy. An optional serrated washer can be placed on the screw head for load distribution. The screws are made from titanium alloy Ti-6Al-4V ELI. SI-Cure features a self-harvesting design that collects autograft tissue as the implant is advanced, eliminating the need for bone grafting. As the SI-Cure is advanced, its helical design pushes bone into the open architecture of the device, allowing the autograft to backfill the length of the implant.
The purpose of this submission is to rebrand the previously-cleared FUSIO Screw Fuze System as the SI-Cure Sacroiliac Joint Fusion System, and to offer more specific and additional indications for use and implant sizes/instrument options.
Alevio Spine was founded in 2015 and is based in Birmingham, Alabama. Leadership brings experience from numerous spine companies, including Baxano Surgical, Benvenue Medical, Centerpulse-Spine-Tech, Integrity Implants, Medtronic, SI-BONE, Spinal Kinetics, SpineGuard USA, Surgalign and Xtant Medical.

NITRO Interbody Fusion Cage
CTL Amedica | NITRO Interbody Fusion Cage, K220513
Submitted February 2022, granted April 2023
Primary predicate: VALEO Spacer System; VALEO II Interbody Fusion Device System, K143518
FYI: NITRO is made entirely of silicon nitride biomaterial, which offers bacteriostatic properties, provides artifact-free imaging and is compatible with all imaging modalities.
Products in the NITRO line feature precisely drilled axial pores to promote capillary action, a pathway for integrated bony through-growth and macro texturing throughout the implants’ expanded surface to increase the area of bony contact and enhance the innate bacteriostatic properties.
Of the broad system, the MATISSE NITRO ACIF Cage will be the first to market, with launch expected in 3Q23.

HAPPE Spine’s INTEGRATE-C Interbody Fusion
HAPPE Spine | INTEGRATE-C Interbody Fusion, K222004
Submitted July 2022, granted April 2023
Primary predicate: Vertera Spine Cohere, K173030
FYI: INTEGRATE-C is reported to be the first interbody fusion cage that is fully integrated with porosity and hydroxyapatite to provide an enhanced healing environment. The interconnected, cancellous porosity promotes bone ingrowth from endplate to endplate. Hydroxyapatite is exposed on all surfaces to promote cell signaling and bone on-growth. INTEGRATE-C is radiolucent and radiovisible for superior intraoperative and postoperative imaging.
The device is presently in limited release to collect clinical data and establish efficacy.

VySpine UniVy OsteoVy-Ti Cervical Interbody Fusion Device
VySpine | UniVy OsteoVy-Ti Cervical Interbody Fusion Device, K223513
Submitted November 2022, granted April 2023
Primary predicate: VySpine UniVy OsteoVy-Ti Cervical IBF System (K221162)
FYI: This device features VySpine’s NanoVy-HA technology in partnership with Promimic. NanoVy-HA is a process in which the 3D-printed UniVy OsteoVy-Ti implant is surface-treated with hydroxyapatite. The NanoVY-HA surface treatment is 20 nanometers thick, offering an alternative to traditional coating that is typically 50 micrometers thick.
The NanoVy-HA coating penetrates the porous lattice structure of the UniVy OsteoVy-Ti implant. A combination of high wettability and surface chemistry, along with optimized nano-roughness, is designed to mediate bioactivity and specific protein adsorption to the implant. These properties may regulate cell behavior and influence tissue regeneration by increasing the osteoblast functions, leading to the building of more bone faster.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




