The following four companies operate in the joint replacement and trauma segments, and received their first orthopedic-focused FDA 510(k) market clearances within 2022.
Joint Replacement
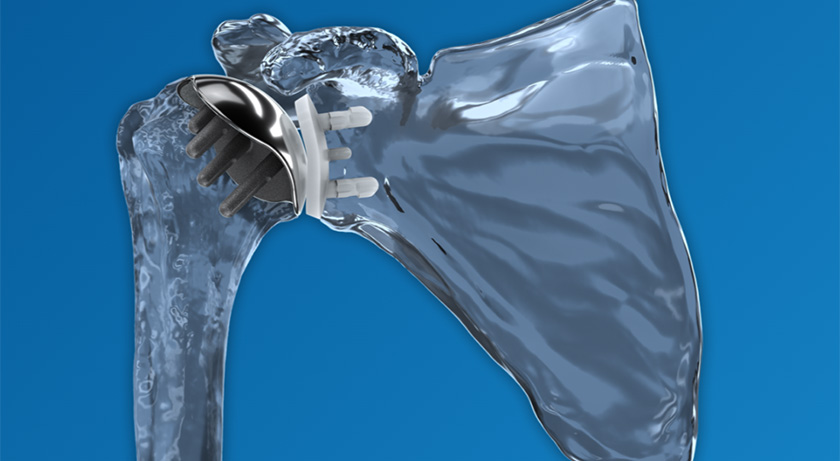
Canary Medical USA | Canary Tibial Extension (CTE) with Canary Health Implanted Reporting Processor (CHIRP) System, K220413
Submitted February 2022, granted June 2022
Primary predicate: Canary Tibial Extension with Canary Health Implanted Reporting Processor (CHIRP) System, DEN200064
- About the product: The Canary Tibial Extension (CTE) with Canary Health Implanted Reporting Processor comprises a tibial extension implant, cloud data management platform, gait parameter software, etc. The CTE and CHIRP System is intended to provide objective kinematic data on a patient’s total knee replacement function. The kinematic data produced by the CTE implant is intended as an adjunct to other physiological measurement tools post-surgical care, while providing additional tibial stability afforded by traditional tibial extensions of similar length. The implanted CTE collects data from internal motion sensors, which is subsequently uploaded to a cloud data management system. The CTE is designed for use with Zimmer Biomet’s Persona Personalized Knee tibial baseplate, to provide additional stability and collect kinematic data to assist the physician in monitoring patient activity following total knee replacement between office visits.
- About the company: Founded in 2012, headquartered in Vancouver, British Columbia. In early 2021, the company received a $20 million venture loan facility. Canary Medical seeks to improve healthcare outcomes through the continuous, passive collection and analysis of data derived primarily from “smart” implantable medical devices that are proposed to report on function, diagnostic information, patient activity, complications and treatment failure for up to 10 years – all without significant patient or physician involvement. Leadership brings experience from roles at BD, CareFusion, DJO/dj Orthopedics, Exactech, Lima USA, Zimmer.
Trauma
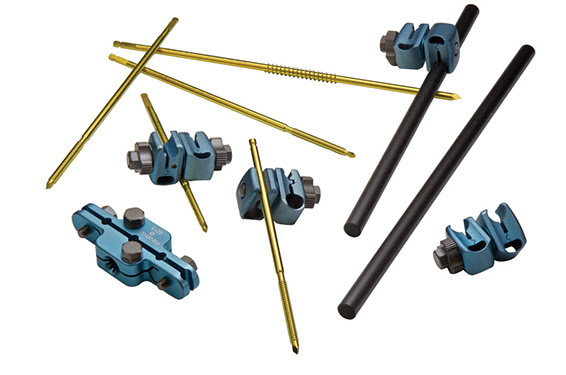
OrthoNovis External Fixation System
OrthoNovis | ONX Large External Fixation System, K213905
Submitted December 2021, granted August 2022
Primary predicate: Renovis Surgical Technologies T 710 Large External Fixation, K110965
- About the product: Comprises clamps, rods and pins that provide the surgeon a broad range of frame construct options for stabilizing or immobilizing fractures of the long bones in tibia and femur, pelvis and ankle, peri-articular and intra-articular fractures of the knee and ankle joints, etc.
- About the company: Founded in 2017, headquartered in Palm Coast, Florida. OrthoNovis employs a direct-to-consumer business model to deliver products at a price below other comparable products in the market. Leadership brings experience from roles at DePuy Acromed, Internal Fixation Systems, Stryker, Synthes Spine, Synthes Trauma.
TECHFIT Digital Surgery | AFFINITY Proximal Tibia System, K220199
Submitted January 2022, granted March 2022
Primary predicate: aap LOQTEQ Proximal Tibia Plate 3.5 System, K132554
- About the product: Consists of anatomical plates and screws for the placement of the proximal tibial condyles, improving the restoration of the original structure. Similarly, in combination with the variable angle technique, it allows for the placement of screws in different configurations providing appropriate support for the correct healing of fractures.
- About the company: Founded 2011, headquartered in Daytona Beach, Florida. TECHFIT’s portfolio of solutions allows for the implementation of digital workflows into any hospital’s musculoskeletal reconstructive surgery program, with no upfront investment and almost 100% variable costs. To date, the company has raised $1,000,000 in a seed round of funding. Leadership brings experience from Industrias Medicas Sampedro, reportedly the largest orthopedic implant manufacturer in Colombia and the largest 3D-printed implant manufacturer in Latin America.
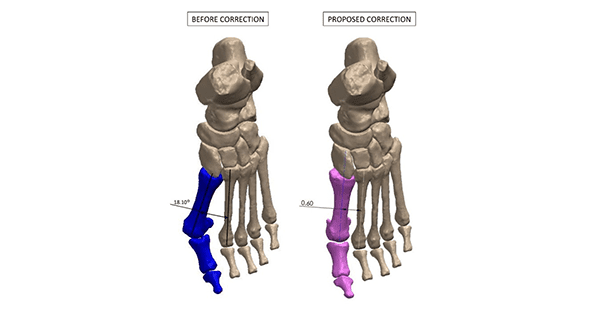
Redpoint Medical 3D | Better Bunion System
Redpoint Medical 3D | Better Bunion System, K220717
Submitted March 2022, granted June 2022
Primary predicate: Materialise Surgicase Guides, K163156
- About the product: The Better Bunion System is intended for use as a surgical instrument to assist in preoperative planning and/or in guiding the marking of bone and/or guide surgical instruments in non-acute, non-joint-replacing osteotomies in the foot and ankle for adult and pediatric patients 12 years of age and older. This reportedly represents the first patient-specific instrumentation (PSI)-based company to gain FDA clearance for patient-specific bone cutting guides for use in foot and ankle reconstructive surgeries. The patient-specific surgical guide system employs proprietary artificial intelligence software (RedPoint Intelligence™) to convert data derived from a CT scan of the patient’s foot and/or ankle into a 3D model, which is then manipulated to resemble the desired correction as prescribed by the surgeon.
- About the company: Founded in 2019, headquartered in Carmel, Indiana.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.