
These clearances span implants and robotic/digital products with application in the spinal segment, from companies both familiar and new.

Stryker Q Guidance System
Stryker | Q Guidance System, K220593
Submitted March 2022, granted May 2022
Primary predicate: Stryker Leibinger Spine Guidance 4.0 Software, K172034
- About the product: When used with Spine Guidance Software, the Q system provides advanced planning and intraoperative guidance designed to enable open or percutaneous computer-assisted surgery. The Spine Guidance Software is reportedly the first spine navigation software to receive clearance from FDA for use with pediatric patients aged 13 and older. Q Guidance delivers planning and navigation through multiple tracking options, software algorithms and smart instrumentation. It features completely redesigned software applications, semi-automatic and automatic segmentation features, gesture recognition and broad compatibility with various types of image sets. When used with the Airo TruCT mobile CT scanner, this ecosystem delivers automatic image registration and pairs high performance tracking capabilities with high intraoperative image quality and scan volume.
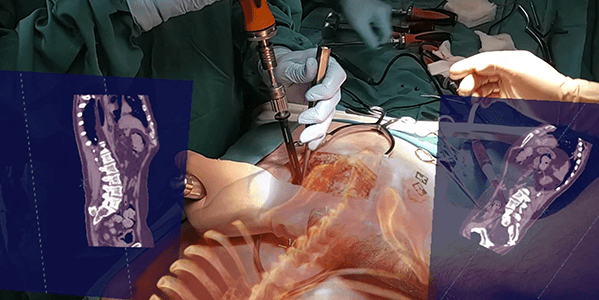
Novarad VisAR Surgical Navigation
Novarad | VisAR, K220146
Submitted January 2022, granted May 2022
Primary predicate: Augmedics xvision Spine System, K190929
- About the product: This augmented reality surgical navigation system is cleared for precision-guided intraoperative spine surgery. VisAR transforms a patient’s imaging data into a 3-dimensional hologram which is visible through an optical visor, and superimposed onto the patient with submillimeter accuracy. The surgeon can focus directly on the surgical objective without looking away at a separate monitor. VisAR is an end-to-end offering with pre-surgical planning, virtual annotations, segmentation and bi-directional image connectivity. It features integrated 2D and 3D immersive navigation views with continuous hologram-to-patient registration. VisAR technology utilizes image visible CT fiducial markers for automatic registration. The operating room setup time is less than 2 minutes. Surgical accuracy is sub 2mm for pedicle screw placement in both open and minimally invasive surgical procedures.

SpineUp Romero Cervical Cage
SpineUp | Romero Cervical Cage, K212358
Submitted July 2021, granted January 2022
- Primary predicate: Medtronic Sofamor Danek DIVERGENCE Anterior Cervical Fusion
About the product: The devices are made of PEEK Optima HA-Enhanced, which enables osteointegration as an alternative to traditional PEEK. This represents SpineUp’s first product range. - About the company: Founded in 2019; headquartered in Hollywood, Florida. Leadership brings experience from Euros, Small Bone Innovations, Spineway.
VySpine | VyPlate Anterior Cervical Plate, K221572
Submitted June 2022, granted July 2022
Primary predicate: Reliance Medical Reliance Anterior Cervical Plate System, K122216
- About the product: Indicated for stabilization of the anterior cervical spine from C2 to C7, employing unicortical screw fixation at the anterior face of the vertebral bodies. The system features plates and screws manufactured from titanium alloy. Its robust yet simple locking mechanism ensures that the bone screws are fully captured within the VyPlate.
- About the company: Established in 2021, headquartered in Tallahassee, Florida. Has developed OsteoVy 3D, a porous lattice structure that can be 3D-printed using various material platforms. Leadership brings experience from roles at Alphatec Spine, Amplify Surgical, Archus Orthopedics, Interpore Cross, LinkSPINE, Pioneer Surgical Technology, Spinal Concepts.

Zavation Medical Products Varisync Plate and Spacer
Zavation Medical Products | Varisync Plate and Spacer, K221049
Submitted April 2022, granted August 2022
Primary predicate: Zavation IBF System, K202305
- About the product: The VariSync Plate is intended for anterior screw fixation to the cervical spine for the treatment of degenerative disc disease, trauma, tumors, deformity, pseudarthrosis, failed previous fusion, spondylolisthesis and spinal stenosis. The VariSync Spacer is an interbody fusion device intended to treat degenerative disc disease of the cervical spine at one level. The spacer is to be filled with autograft or allogenic bone graft comprising cancellous and/or corticancellous bone graft in skeletally mature patients. These devices are intended to be used with supplemental fixation such as the Zavation VariSync Plate, Zavation Midline Plate, Zavation EZ Plate or Zavation Cervical Plate Systems. When used with the VariSync Plate, the assembly takes on the indications of the VariSync Spacer, with the VariSync Plate acting as the supplemental fixation. Varisync has been tested and approved for both the independent and synchronized use of its plate and spacer components. This allows surgeons to use these plate and spacer options together as a system or pair individual components with any of Zavation’s existing cervical portfolio options.
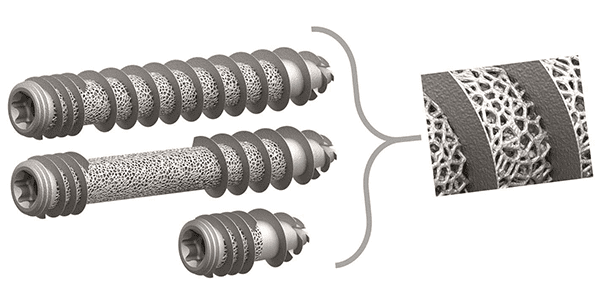
Cutting Edge Spine T-FIX 3DSI Joint Fusion
Cutting Edge Spine | T-FIX 3DSI Joint Fusion, K214123
Submitted December 2021, granted June 2022
Primary predicate: Orthofix FIREBIRD SI Fusion, K203138
- About the product: The 3D-printed T-FIX 3DSI Joint Fusion System is part of the EVOL-SI Fusion portfolio, which is designed to treat dysfunctions of the sacroiliac joint. The device is cleared for multiple approaches: Lateral, S2Al (S2 Alar Iliac), Open Posterior Bridging and Transgluteal Lateral. The system’s trabecular technology is designed to promote optimal osseointegration, positively impacting fusion and fixation.
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics) | KEOPS Balance Analyzer 3D Surgery Planning Software, K213975
Submitted December 2021, granted May 2022
Primary predicate: Nemaris Surgimap, K141669
- About the product: Balance Analyzer 3D is spinal realignment planning software that uses medical imaging of the patient’s spine. Since receiving approval under the CE Mark in 2014, it has been an integral part of the i-plan surgical planning solution marketed by SMAIO within its comprehensive i-kontrol platform that allows surgeons to treat spinal pathologies. i-kontrol incorporates planning, implants and related services. Obtaining FDA 510(k) clearance is a key element of a partnership and licensing agreement signed in 1Q22 between SMAIO and NuVasive to further develop SMAIO’s surgical planning solutions and to support the innovation pipeline and commercialization efforts.

Osseus Pisces-SA Standalone ALIF Interbody
Osseus | Pisces-SA Standalone ALIF Interbody, K213935
Submitted December 2021, granted May 2022
Primary predicate: Globus Medial HEDRON Lumbar Spacer, K191391
- About the product: Pisces-SA can be used with both bone screws and alternative fixation bone anchors allowing for increased intraoperative flexibility. Biomechanical testing has indicated that Pisces-SA anchors provide better expulsion resistance than the competition and perform comparably with traditional screw-based standalone ALIF constructs in stabilizing injured spinal segments. Pisces-SA is reportedly the first of its kind to provide this level of expulsion resistance and segmental stabilization using an alternative fixation method. The platform integrates a highly-porous 3D-printed interbody with anatomical morphology designed for full osseointegration with streamlined instrumentation to facilitate a minimally invasive approach.
CTL Amedica | CTL Amedica Navigation Instrument System, K213491
Submitted November 2021, granted May 2022
Primary predicate: Orthofix Navigated Instrument System, K172115
- About the product: The system features manual surgical instruments adaptable for use with third-party navigation, that are designed to assist surgeons in locating anatomical structures in either open, minimally invasive or percutaneous procedures for preparation and placement of pedicle screws. The instruments are manufactured from stainless steel, and the system is compatible with CTL Amedica’s RAPHAEL™ Pedicle Screw, PICASSO II™ MIS Spinal System and the TAURUS™ Pedicle Screw. The third-party navigation systems are indicated for any medical condition in which the use of stereotactic surgery may be appropriate and where reference to a rigid anatomical structure, such as vertebra, can be identified relative to a CT- or MR-based model, fluoroscopy images or digitized landmarks for the anatomy. The surgical imaging technology provides surgeons visualization for complex and MIS procedures and aids in establishing trajectory during advanced surgical procedures. These navigation systems provide surgeons with access to real-time, multi-plane 2D and 3D images, providing visual representation of hardware placement.

Accelus Toro Lateral Interbody Fusion
Accelus | Toro Lateral (Toro-L) Interbody Fusion, K213355
Submitted October 2021, granted March 2022
Primary predicate: Integrity Implants Toro-L Interbody Fusion System, K203038
- About the product: Toro-L is a biplanar expandable lateral implant designed for a minimal insertion profile and maximum bone graft delivery directly through the inserter. Toro-L features an insertion profile that is 14mm wide, expanding to the implant’s full width of either 21mm or 24mm before further expanding to the surgeon’s desired height of up to 16mm. The insertion height and fully expanded height are relative to the lordosis of the implant. The implant will initially be offered with a 10-degree lordotic option during alpha launch with 5-degree and 15-degree lordotic options offered upon full commercial launch. The implant has 3D-printed endplates, which have a roughened surface where the implant interfaces with bone due to the additive manufacturing process. In addition, the Toro-L system is equipped with an inlet retractor. Although the inlet retractor has a feature-rich split-tube design, the Toro-L system was created such that the surgeon should never have to open the tube to perform the surgery. Unique and proprietary disc removal instrumentation, along with traditional curettes, rasps, scrapers, kerrisons, and pituitaries, give the surgeon the ability to fully evacuate the disc space and accommodate the full-width footprint of the expanded Toro-L implant. The system also features an optimized post-pack graft delivery method that is fast and efficient.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




