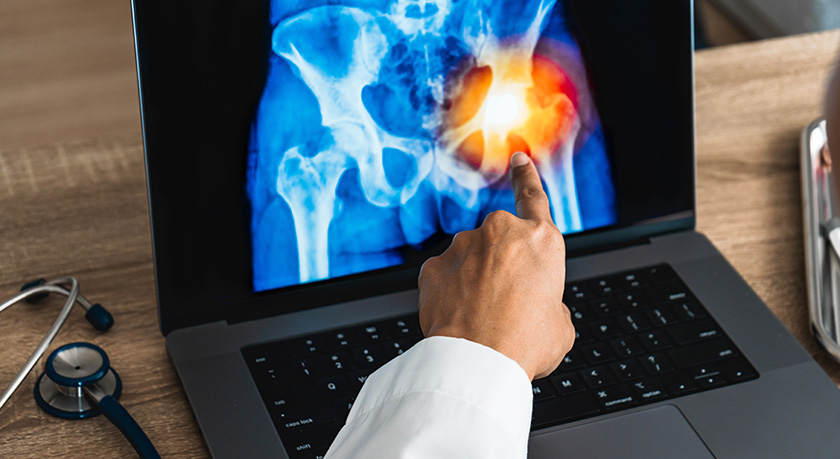
Exactech announced that FDA granted a Breakthrough Device Designation for JointMedica’s Polymotion Hip Resurfacing System.
Exactech, a minority shareholder of JointMedica, is collaborating to deliver the next generation of hip resurfacing to the global market, and holds exclusive global distribution rights to the product.
The Polymotion hip is designed to offer the biomechanical benefits of hip resurfacing while eliminating metal-on-metal articulating surfaces. The system utilizes advanced polyethylene and titanium manufacturing technology and aims to be a better solution for younger, more active patients.
“Through this deep collaboration, Exactech and JointMedica are well-positioned to bring the Polymotion Hip Resurfacing System to the global market,” said Jeff Binder, CEO and Chairman of the Board of Exactech. “Polymotion will complement Exactech’s Alteon hip arthroplasty portfolio, comprised of substantial advancements in design and materials to time-tested solutions.”