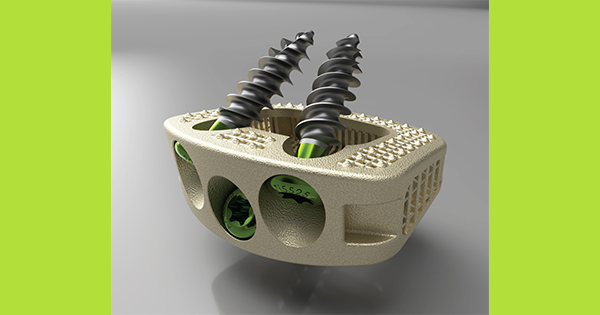
Innovasis is the first company to receive FDA 510(k) clearance for a Stand-Alone ALIF System with an HAnano Surface® modification. Product launch is underway.
The Innovasis AxTiHA® Stand-Alone ALIF intervertebral fusion device is used to facilitate fusion in the lumbar spine and is inserted using an anterior lumbar interbody fusion procedure. The crystalline hydroxyapatite (HA) surface particles mimic bone tissue through shape, composition and structure.
The AxTiHA Stand-Alone ALIF implant also features a large graft cavity and open geometric structure, providing increased volume for autograft loading and bone through-growth. The device includes integrated fixation by way of three converging bone screws and optional screw anti-backout locking clip.
Source: Innovasis
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.