
This month’s recap shares the last of the companies that received their first clearances in 2021 for products with spinal, trauma, robotics/digital and sports medicine applications. The entities span the globe with locations in Belgium, South Africa, South Korea, Switzerland, the U.K. and the U.S.
Spine
Intelivation Technologies | Golden Isles Pedicle Screw, K212185; submitted July 2021, granted August 2021
- Primary predicate: Amedica Preference Elite Pedicle Screw, K162160
- About the product: Spinal fixation system comprising a variety of components including pedicle screws, modular head bodies and various types and sizes of rods; designed to provide superior strength at the tulip and screw interface. Launched in 3Q21.
- About the company: Established in 2020, based in St. Simons Island, Georgia. Other products include plates and staples for various trauma applications, as well as cervical and lumbar cages, a cervical plate, etc. Leadership brings experience from Dio Medical, GenOssis, Globus Medical, Integra, Synthes Spine.

JMT EDEN PEEK Cage
JMT | EDEN PEEK Cage, K201793; submitted June 2020, granted September 2021
- Primary predicates: L&K Biomed Lumbar Interbody Fusion Cage, K181380; U&I Corporation Velofix Interbody Fusion, K171749
- About the product: Cervical interbody fusion device (ACIF type) which may be implanted as a single device via an anterior approach; lumbar Interbody Fusion Device (PLIF type) which can be implanted bi-laterally via a posterior approach.
- About the company: Founded in 2006, based in Gyeonggi-do, South Korea. Other products include an interference screw, washer/screw and double arm needle for sports medicine applications, an expandable spinal cage, various catheter systems for pain management, etc.
LESspine Innovations | Inspan ScrewLES Fusion System, K213266; submitted September 2021, granted December 2021
- Primary predicate: SpineFrontier Vega SPAN Spinous Process Plate, K102020
- About the product: Previously cleared as the primary predicate product. Posterior non-pedicle supplemental fixation system intended for use in the non-cervical spine.
- About the company: Founded in 2006, located in Malden, Massachusetts. A KICVentures Group portfolio company focused on research and development of Less Exposure Surgery (LES) technologies that support faster recovery in an outpatient setting. Leadership also holds roles at AxioMed, FacetFuse, Inspan, MISquito, NanoFUSE Biologics, Sacrix, Sagitechnology.
NovApproach Spine | OneLIF Intervertebral Body Replacement, K211769; submitted June 2021, granted September 2021
- Primary predicate: Medtronic Sofamor Danek DIVERGENCE Anterior/Oblique Lumbar Fusion System, K150135
- About the product: The company’s patent-pending anterior approach enables the surgeon to reach all lumbar spinal levels without a long incision or changing the patient’s position on the table. OneLIF Interbody Fusion will complement the SupineATP technique in even highly challenging cases.
- About the company: Founded in 2017 in Alachua, Florida. Leadership brings experience from time at Exactech, Stryker, Zimmer Biomet.
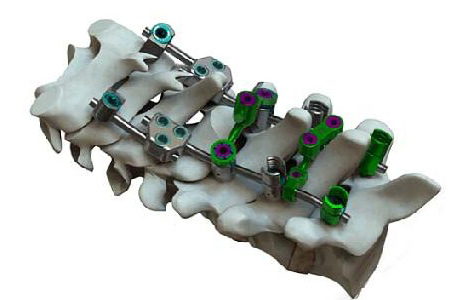
VySpine VySpan
VySpine | VySpan PCT System, K213394; submitted October 2021, granted December 2021
- Primary predicate: Reliance Medical Systems’ Reliance Posterior Cervical-Thoracic System, K142867
- About the product: Various screw and hook options, multiple transition rods and revolutionary crosslink versatility, and a variety of implant options for the thoracic spine; includes fixed and polyaxial head styles, each with a reduction option, that can be paired with either standard or smooth shank bone screws and a variety of hooks and rods. An assortment of rod-to-rod and cross connectors includes a novel head-to-head double joint cross connector, as well as three- and four-point cross connectors for versatility.
- About the company: Established in 2021, headquartered in Tallahassee, Florida. Two other spinal implants cleared during 2021 (ClariVy Cervical IBF and VyWasher Buttress System). Company has developed OsteoVy 3D, a porous lattice structure which can be 3D-printed utilizing various material platforms. Leadership brings experience from roles at Alphatec Spine, Amplify Surgical, Archus Orthopedics, Interpore Cross, LinkSPINE, Pioneer Surgical Technology, Spinal Concepts.
Trauma
Bespa Global | BESPA Charcot System, K202326; submitted August 2020, granted November 2021
- Primary predicate: Wright Medical Salvation Beams and Bolts System, K140741
- About the product: Medial and lateral segmental columns to align and secure various bones in the foot.
- About the company: Service provider offering an intermediary between medical device companies and healthcare professionals; founded 2005 and based in Dallas, Texas. Among other offerings, Bespa develops technologies internally with its surgeon and engineering teams. Leadership includes a practicing orthopedic surgeon and others with sales/marketing/distribution experience for OEMs such as Biomet, Osteonics, Spinal Dimensions, Stryker.
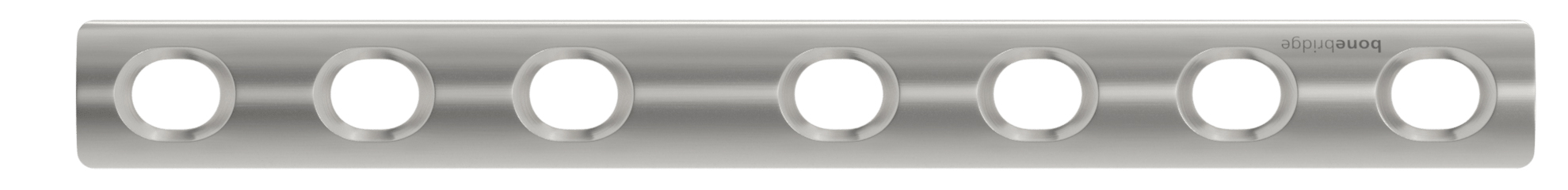
Bonebridge Osteosynthesis Plating System
Bonebridge | Osteosynthesis Plating System (including the TRIFT Tubular System), K203002; submitted October 2020, granted October 2021
- Primary predicate: Synthes One-third Tubular Plate 3.5mm, K011335
- About the product: Indicated for treatment of smaller fractures of long bones such as humerus, fibula, and ulna.
- About the company: Founded in 2018, based in Zug, Aargau, Switzerland. Markets orthopedic trauma implants that are reduced in complexity, including devices for the clavicle, proximal humerus, distal radius, distal ulna, proximal tibia, etc.

Innovate Orthopedics Quick Start Screw
Innovate Orthopaedics | Quick-Start Screws, K212547; submitted August 2021, granted December 2021
- Primary predicate: Smith & Nephew RCI Fixation Screws, K992945
- About the product: Quick-Start Screws are indicated for interference fixation of soft tissue grafts and/or bone-tendon-bone grafts for ligament reconstruction such as anterior/posterior cruciate ligament, medial collateral, lateral collateral, posterolateral corner and medial patellofemoral reconstructions.
- About the company: Established 2014, located in Slaithwaite, Huddersfield, England. Has also developed a Quick-Start Guidewire and Quick-Start Hex Driver. Aside from the U.K., Innovate Orthopaedics also exports products to New Zealand and Israel, and is negotiating to enter markets in South Africa and Australia. Leadership brings experience from a variety of roles at Smith+Nephew.
Sports Medicine

Ortho-Design VersaTap
Ortho-Design | VersaTap VersaLat DueLock VersaTi MiniTi, K212381; submitted August 2021, granted December 2021
- Primary predicate: Parcus Medical Suture Anchors, K120942
- About the product: Self-tapping suture anchor mostly used as a medial row anchor in rotator cuff repair surgery, designed to combine the advantages of both PEEK and titanium. The titanium tip allows for self-tapping ability, while the majority of the anchor is manufactured from PEEK which minimizes post-operative imaging effects.
- About the company: Founded in 2016, headquartered in Die Wilgers, Pretoria, South Africa. Founded and managed by two biomedical engineers with international experience in biomechanics, mechanical and mechatronic engineering.
Robotics/Digital
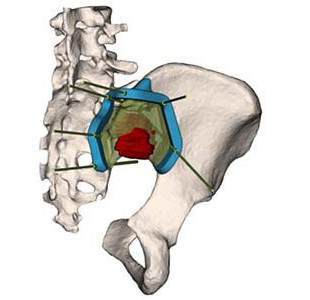
3D-Side 3D-Cut
3D-Side | 3D-Cut, K212237; submitted July 2021, granted November 2021
- Primary predicate: 3D Systems VSP Orthopedics, K190044
About the product: Patient-matched, additively manufactured single-use surgical instrument (PSI). Based on preoperative planning, the instruments are intended to assist physicians in guiding the marking of bone and guiding surgical instruments in bone tumor resection surgery, excluding joint replacement surgeries. The target locations are femur, tibia and pelvis, including the sacrum. Company’s first 510(k) clearance. - About the company: Founded 2015, located in Mont Saint Guibert, Belgium. Developer of the Customize platform to connect surgeons, patients and medical device companies to optimize and streamline communication, creation and supervision of the production of patient-specific medical devices.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




