
This month’s roundup features one device from each of the major segments, including one lesser-known name: Denmark-based Radiobotics, granted clearance for its artificial intelligence-based analysis tool designed to enhance accuracy during radiographic knee osteoarthritis diagnosis.
Joint Replacement
Shoulder Innovations | InSet Reverse Shoulder Arthroplasty System, K210533; submitted February 2021, granted August 2021
- Primary predicate: Biomet Comprehensive Reverse Shoulder, K080642
- About the product: Convertible humeral platform demonstrating best in class fixation, the only true fully convertible humeral inlay system and minimally invasive bone preservation. Includes the artificial intelligence-enabled 3D PreView Software Planning solution to support precise implant selection and placement, as well as a broad range of implant types representing six different products, integrated into one instrument tray, optimized for ASC environments.
- About the company: Established in 2015, based in Holland, Michigan. From 2015 through 2020, the company has raised $25.9 million over four rounds. Leadership and staff bring experience from roles at Conventus Orthopaedics, DePuy Orthopaedics, Kinetikos Medical, Magnesium Development Company, Tornier and Zimmer Biomet.
Spine
Kleiner Device Labs IMAGE | Kleiner KG2 Surge flow-thru interbody system, K211474; submitted May 2021, granted September 2021
- Primary predicate: Osseus Aries-TC and Aries-TS system, K181347
- About the product: 3D-printed titanium, featuring a diamond lattice porous structure to facilitate bone ingrowth. The system maximizes total bone graft delivery volume, better distributes graft bilaterally into the intervertebral disc space and streamlines the implant delivery, positioning and grafting process for TLIF and PLIF spinal fusion. An I-beam architecture and ramp design allow unrestricted flow to bilaterally fill and integrate the implant into the prepared disc space.
- About the company: Founded in 2017, headquartered in Incline Village, Nevada. Leadership has experience from time at Aegis Spine, Biomet Spine, Codman & Shurtleff (a J&J company), Lanx, VertiFlex and Zimmer Biomet.
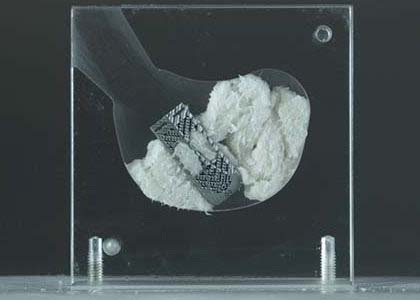
Kleiner KG2 Surge
Trauma
The Orthopaedic Implant Company | DRPx Locking Distal Radius Plate, K202971; submitted September 2020, granted May 2021
- Primary predicate: Orthopaedic Implant Company OIC Distal Radius Plating System, K123832
- About the product: Reportedly the only distal radius plating system that features an enhanced ergonomic design to meet the technique preferences of surgeons while significantly increasing cost savings to help improve the financial viability of ambulatory surgery centers and hospitals. Designed with Type II anodized titanium, the DRPx features pre-contoured anatomic plates, a distal window for graft and/or fracture visualization and a spectrum of targeting instrumentation to facilitate precise and fast drilling trajectories.
- About the company: Established in 2010, located in Reno, Nevada. Leadership experience includes time at companies like Devise Orthopaedics and Smith & Nephew
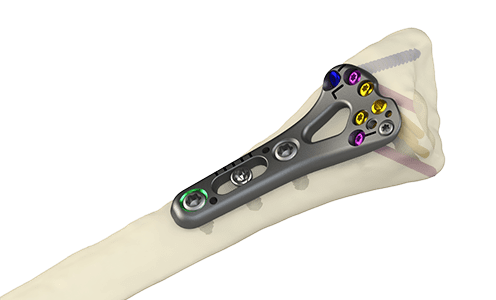
DRPx Locking Distal Radius Plate
Sports Medicine
Trice Medical | mi-Eye 3 Needlescope with Cannula, mi-Tablet 3, K212556; submitted August 2021, granted September 2021
- Primary predicate: Trice Medical mi-eye 2, mi-eye 2 monitor, K162475
- About the product: 25-degree mi-eye 3 needlescope with horizon leveling technology, offering unparalleled views and maneuverability in arthroscopic and endoscopic procedures from a single-use, single-hand device. Used with the proprietary high-resolution mi-tablet 3, the technology takes minimally invasive operative and diagnostic capabilities in small joints and patient outcomes to a new level. The platform integrates the latest technology with 8-in-1 capabilities: image horizon leveling, high-resolution image sensor, integrated camera with LED illumination, single-hand operation, image management, seamless workflow with hospital information management systems, the ability to manipulate the viewing angle with horizon preferences and portability. The new mi-eye 3 needlescope camera performs the same as a reusable but in a small disposable form.
- About the company: Established in 2012, located in Malvern, Pennsylvania. Trice has raised a total of $88.4 million in funding over seven rounds from 2012 through 2021, including an investment from Bioventus. In 2019, Trice acquired SegWAY Orthopaedics, provider of systems to treat the extremities such as the Endoscopic Carpal Tunnel Release device. Further, in 2021, the company purchased Tenex Health, provider of minimally invasive ultrasonic technology to treat chronic pain in soft tissue and bone. Trice leadership has served the orthopedic industry at companies like Covidien, MAKO Surgical, OMNI, Smith & Nephew, Tenex and Wright Medical.

mi-Eye 3 Needlescope
Orthobiologics
Royal Biologics | Maxx-PRP Platelet and Plasma Separator for Bone Graft Handling, BK210563; submitted March 2021, granted April 2021
- About the product: Maxx-PRP is a next-generation concentration device that can concentrate autologous whole blood for rapid preparation of platelet-rich plasma to use point of care for mixing with autograft and/or allograft to improve handling characteristics. Maxx-PRP system offers “customizable formulations” of platelet-rich plasma, allowing clinicians to treat a variety of conditions in orthopedics, sports medicine and regenerative medicine. Maxx-PRP also allows users to perform two concentration cycles in one device, in under 10 minutes. This novel technology sets a new bar in the orthopedic and regenerative medicine industry for concentration times, customizable formulations and efficiency.
- About the company: Founded in 2015 and located in Hackensack, New Jersey. Leadership experience includes roles at Alphatec Spine, Integra LifeSciences, Osiris Therapeutics, Smith & Nephew and Synthes. Within 2021, the company entered into a distribution agreement whereby Vilex will market and distribute the company’s portfolio of Autologous and Live Cellular (ALC) therapies for lower extremity orthopedic procedures. Royal Biologics has now launched three novel systems in its ALC portfolio that provide an efficacious and cost-benefit advantage to clinicians and hospital systems.

Maxx-PRP Platelet and Plasma Separator
Robotics/Digital
Radiobotics | RBknee, K203696; submitted December 2020, granted August 2021
- Predicate device: IB Lab KOALA image-processing software, K192109
About the product: An artificial intelligence-based analysis tool aimed at improving the efficiency and accuracy of radiographic knee osteoarthritis (OA) diagnosis. Intended for use in an orthopedic or a radiological setting, where the x-ray is read in order to determine the diagnosis and treatment of OA. RBknee can identify presence or absence of osteophytes, subchondral sclerosis, and joint space narrowing using proprietary machine learning algorithms. Furthermore, RBknee can accurately measure the joint space width in both compartments of the knee. RBknee is also CE Marked. - About the company: Founded in 2017, located in Copenhagen, Denmark. Raised $4 million in funding over five rounds from 2018 to 2020. Has also developed RBhip to automatically detect findings relevant for radiographic hip osteoarthritis, together with automatic measurement of common angles. Leadership brings experience from Embody Orthopaedic. Earlier this year, Radiobotics unveiled its first subsidiary, Radiobotics Inc. in the U.S.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




