
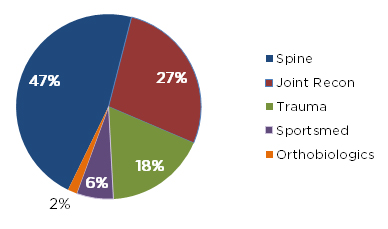
Of the 62 devices and systems that gained FDA 510(k) clearance by the orthopedic panel in October, spine once more represented the majority with 29 clearances, followed by joint replacement (17), trauma (11), sports medicine (4) and orthobiologics (1). Exhibit 1 below depicts each segment’s share for the month.
Exhibit 1: FDA 510(k)s from October 2019, by Market Segment
This month, we’ll focus on 10 products evenly split between the joint replacement and spine segments, half of which are the company’s first orthopedic-related clearance such as FX Shoulder USA, Amplify Surgical and restor3d. Others like Exactech, Smith+Nephew and THINK Surgical have been around for a while, and are called out for interesting technologies or line expansions.
Hip
Exactech | Alteon Modular Dual Mobility System, K190890
- Submitted April 2019
- Primary predicate: Corin Trinity Dual Mobility System (K170359)
- Liners and inserts manufactured from CoCr alloy and Ultra-High Molecular Weight Polyethylene containing vitamin E, respectively
- Can be used in primary or revision patients who are at a high risk of hip dislocation
- Intended for press-fit fixation
- Joining others with dual mobility systems, including Aston Medical, Corin, LimaCorporate, Serf, Smith+Nephew, Stryker, Waldemar Link and Zimmer Biomet
Smith+Nephew | OR3O Dual Mobility System, K191002
- Submitted April 2019
- Primary predicate: Smith+Nephew POLARCUP Dual Mobility System (K110135)
- Indicated for primary and revision hip procedures
- Modular OR3O Liners and Inserts are to be implanted without bone cement; mating components may be indicated for use without bone cement
- Designed with a small-diameter femoral head that locks into a larger polyethylene insert to increase stability and improve range of motion
- Employs OXINIUM DH (Diffusion Hardened) bearing surface for its liner, specifically intended for hip arthroplasty and designed to increase the depth of hardening in a device through a patented technology process
- Will be the largest product launch in the company’s history
Knee
Implanet | Madison Total Knee System, K192084
- Submitted August 2019
- Primary predicate: Stryker Scorpio NRG Knee (K915192, K071991)
- First knee device cleared for the company in the U.S.
- Indicated for primary and revision procedures
- Components are indicated for cemented fixation only
- Clearance supported by clinical results, including data collected over seven years and 17,000 implants
- Clearance is a key component in Implanet’s partnership with KICO Knee Innovation Company to distribute the device initially in the U.S. and Australia, as signed in 2018
THINK Surgical | TSolution One Total Knee Application, K191369 (main image)
- First U.S. clearance for knee; formerly only available in the U.S. for hip applications
- Submitted May 2019
- Primary predicate: THINK Surgical TSolution One Surgical System Model 210 (K170430)
- Employs TPLAN 3D planning workstation and TCAT, a computer-assisted tool that executes the pre-surgical plan
- Uses diagnostic images of the patient specifically to assist the physician with preoperative planning and to provide orientation and reference information during intraoperative procedures
- The robotic surgical tool, under the direction of the surgeon, implements the pre-surgical software plan
- Compatible with Zimmer Persona Knee system
Shoulder
FX Shoulder USA | 32mm Glenosphere and Humeral Cup, K192206
- First FDA 510(k) clearance
- Submitted August 2019
- Primary predicates: FX Solutions’ Humelock II Reversible Shoulder (K150488), Humelock Reversed Shoulder (K162455) and Humeris Shoulder (K163669)
- Current filing presents new components for predicate devices when used for a reverse shoulder construct
- About the company:
- Based in Dallas, Texas as the U.S. subsidiary of France-based FX Solutions, which is a Viscogliosi Bros. portfolio company
- Parent company acquired shoulder assets of Small Bone Innovations in 2010
Spine
Amplify Surgical | DualX Lumbar Intervertebral Body Fusion Device, K192434
- First FDA 510(k) clearance under new company brand
- Submitted September 2019
- Primary predicate: Innovasive DualX Lumbar Intervertebral Body Fusion Device (K181397)
- Purpose of submission is to modify layout of sterilization trays of DualX to include non-sterile implants to be sterilized by the end-user, with no changes to the implants and instruments previously cleared under predicate device (Innovasive rebranded as Amplify in early 2019)
- Intended for use with supplemental fixation and autograft and/or allogenic bone graft
- Indicated for unilateral or bilateral implantation
- Expands sequentially in lateral and then vertical directions
- About the company:
- CEO has executive-level experience at Stryker, VertiFlex, NuVasive Specialized Orthopedics (formerly Ellipse Technologies), CTL Medical and IntuitiveX, a life science incubator
CarboFix Orthopedics | CarboClear VBR System, K192214
- Submitted August 2019
- Primary predicate: Eden Spine Giza Vertebral Body Replacement ( K112429)
- Made of carbon fiber reinforced polyetheretherketone (CFR-PEEK), with titanium alloy endplates
- Intended for use with supplemental fixation; use of allograft or autograft is optional
- Device represents an expansion of CarboClear material use; potential advantages of carbon fiber devices include enhanced radiation therapy planning abilities and enhanced follow-up abilities due to artifacts-free imaging (unlike titanium)
CMF Medicon Surgical | MediExpand TL Expandable VBR System, K190349
- First FDA 510(k) clearance for U.S. subsidiary
- Submitted February 2019
- Primary predicate: summary not available
- Developed for reconstruction of the thoracic and lumbar spine after single or multilevel corpectomies as a result of tumors
- About the company:
- U.S. subsidiary of Germany-based Medicon eG, manufacturer of instruments and implants for a variety of medical applications
GENOSS (FIMS Mfg. on behalf of GENOSS) | 3D Cage, K180347
- First orthopedic-related FDA 510(k) clearance (previous clearances were dental devices)
- Submitted February 2018
- Primary predicate: DePuy Synthes (EIT) EIT Cellular Titanium PLIF Cage, TLIF Cage, K170503
- Indicated for treatment of degenerative disc disease at one or two contiguous levels with supplemental fixation and autograft bone
- Open architecture truss designed to provide structural support with open space throughout the implant for bone growth and fusion
- Provided sterile, constructed from titanium alloy using Selective Laser Melting, custom-printed for each patient
- About the company:
- Korea-based, founded in 2004
- Other products, not FDA-cleared, include the Integral Cervical Cage, Anterior Bone Bridging Cage and a lumbar pedicle screw
restor3d (Additive Device, doing business as restor3d) | ADI Cervical Interbody Fusion Device, K191812
- First FDA 510(k) clearance
- Submitted July 2019
- Primary predicate: Spinal Elements Crystal, K073351
- Indicated to treat degenerative disc disease via an anterior approach using autogenous bone and supplemental fixation
- Comprises a single, continuous piece of titanium alloy fabricated via additive manufacturing
- Has a porous structure throughout the implant body, a circular window for the packing of graft material, a threaded hole for insertion, teeth on the surfaces of the perimeters to aid expulsion resistance and side windows on the walls of the implant
- About the company:
- Founded in 2017, based in Durham, North Carolina
- Co-founded by Ken Gall, Professor of Mechanical Engineering and Duke University, and veteran in the study of materials processing, structure and mechanical properties
- Entered into an exclusive development and licensing agreement with SeaSpine; the two will leverage their specialties to develop interbodies for a wide range of surgical approaches, while driving both clinical and economic advantages
- SeaSpine/restor3d products slated to launch in 2H20
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




