
Startups and small familiar companies take the spotlight in this month’s recap, which includes an approach to head off bacterial contamination, a first-of-its-kind additively manufactured pedicle screw and a way to secure bone fixation during osteotomies. The roundup also highlights a suture-based method for improving tendon-to-bone repairs and an expanded indication for Bone Solutions’ magnesium-based bone graft substitute.
Joint Replacement
 Onkos Surgical | ELEOS Proximal Tibia with NanoCept, K252920
Onkos Surgical | ELEOS Proximal Tibia with NanoCept, K252920
Submitted September 2025, granted October 2025
FYI: This marks the first 510(k) clearance for the NanoCept Antibacterial Technology since the original De Novo authorization was granted in April 2024.
NanoCept offers a proactive approach against intraoperative bacterial contamination. In preclinical studies supporting the original De Novo, NanoCept demonstrated up to a 99.999% kill rate of bacteria that are commonly found in the O.R.
The ELEOS Proximal Tibia with NanoCept Antibacterial Technology is part of the company’s ELEOS Limb Salvage System, which offers comprehensive reconstruction options for patients with significant bone loss due to cancer, trauma or previous surgical procedures.
Spine
 ALLUMIN8 | A8 INTEGR8 Pedicle Screw, K242827
ALLUMIN8 | A8 INTEGR8 Pedicle Screw, K242827
Submitted September 2024, granted October 2025
FYI: According to Allumin8, the product is the first 5.5mm diameter pedicle screw produced through additive manufacturing. The system represents the foundation of what ALLUMIN8 calls Therapeutic Hardware, a platform of next-generation orthopedic and spine implants designed to integrate advanced surface architecture and precision engineering with therapeutic delivery to treat nearly 300 disease states wherein bone contributes to spinal fusion failures.
The company’s approach leverages additive manufacturing to enable Gaussian topography features designed to support osseointegration and future compatibility with localized biologic delivery concepts under research.
Results of mechanical evaluations demonstrated high durability and resistance to fatigue at critical transition zones.
This is the company’s first FDA-cleared device, and international distribution plans are already underway in the U.S., India, Dubai, Australia, Taiwan and Japan.
Trauma
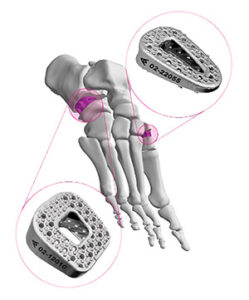 Auxano Medical | ARKEO Wedge Fixation, K251791
Auxano Medical | ARKEO Wedge Fixation, K251791
Submitted June 2025, granted September 2025
Primary predicate: Ames Medical Prosthetic Solutions OsteoSinter EVANS and COTTON wedge and related accessories, K240461
FYI: Indicated for use in Evans and Cotton osteotomies, the ARKEO Wedge integrates an anatomical design with the pillared microstructure of Auxano’s in-growth technology to provide a secure fixation interface at the site of correction.
The system is designed to optimize fixation and graft integration through anatomical fit, open architecture, controlled stability and intuitive instrumentation. Supplied non-sterile in a steam-sterilizable tray, the ARKEO system includes a Hintermann distractor, k-wires, osteotomes, mallet, slap hammer, implant trials, inserter and an impactor.
Sport Medicine
 SutureTech | RapidFix All-Suture Dual Anchor, K250095
SutureTech | RapidFix All-Suture Dual Anchor, K250095
Submitted January 2025, granted September 2025
Primary predicate: Stryker All Suture Anchors, K120509
FYI: RapidFix is intended for fixation of soft tissue to bone in a variety of orthopedic procedures.
The device is a 100% suture-based internal fixation system engineered to create secure tendon-to-bone repairs while reducing procedural steps and surgical complexity.
This is the company’s first FDA-cleared device, and limited launch will occur before the end of the year.
Orthobiologics
 Bone Solutions | Mg OSTEOINJECT, Mg OSTEOREVIVE and Mg OSTEOCRETE, K251522
Bone Solutions | Mg OSTEOINJECT, Mg OSTEOREVIVE and Mg OSTEOCRETE, K251522
Submitted May 2025, granted October 2025
FYI: This expanded clearance allows for use of the technologies in pediatric patients aged six years and older, and highlights the adaptability of Bone Solutions’ proprietary magnesium-based platform, particularly in the Sclerograft procedure — a minimally invasive outpatient technique for treating unicameral bone cysts.
The procedure involves chemical curettage of the cyst wall with doxycycline, followed by injection of Mg OSTEOINJECT low-viscosity bone graft substitute. Mg OSTEOINJECT is designed to fill bone voids or defects in the extremities or pelvis resulting from tumor or cyst removal, trauma or subchondral cystic changes due to osteoarthritis.
Mg OSTEOCRETE and Mg OSTEOINJECT are magnesium oxide-based formulations that initiate a crystallization reaction with phosphate compounds to deliver optimal osteoconductivity.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




