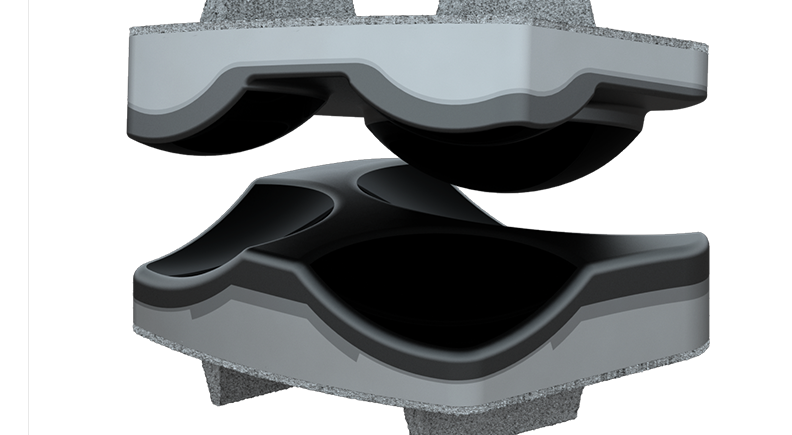
Motion-preserving technologies are driving a new era of designs for spine implants. From artificial discs to dynamic stabilization systems, these solutions aim to maintain the spine’s natural biomechanics while effectively treating spinal conditions.
Device developers are prioritizing minimally invasive approaches and creating implants with smaller profiles and modular designs for easier surgical delivery. New technologies are meeting the growing interest in solutions that are shifting from rigid fixation to devices that match the complexity of spinal movements.
While spinal fusion is currently the standard for treating vertebrae instability, motion preservation signals the future of spine care.
Consider that Centinel Spine, which divested its fusion business in 2023 to focus on developing motion preservation technology and is the only company with FDA approval for both cervical and lumbar total disc replacement systems, announced nearly $33 million in worldwide revenue for the second quarter of 2025, 47% more than the previous year.
The company also reported a prodisc user-base of nearly 850 U.S. surgeons, up nearly 43% from the year before.
Centinel Spine CEO Steve Murray said the quarterly results reflect the continued strength and momentum of the prodisc business. It also shows the promise that motion-preserving devices represent.
Growing Interest
Individual companies are pursuing their own paths to motion-preservation success, but industry observers agree on a few key factors that are driving demand.
First, patients have increased expectations of better function following spine surgery, said Josh Butters, CEO of Synergy Spine Solutions.
“Do they want to have a fusion performed and potentially need to have adjacent segment surgeries 10 years down the road? Or do they want to undergo a procedure that can preserve motion, function and potentially delay or even eliminate the need for those extra surgeries?” he said. “To me, it’s an obvious choice. People want to move normally, and they want to restore their spine’s function, alignment and motion.”
Highridge Medical, in addition to its well-established Mobi-C artificial cervical disc, has licensed the U.S. rights of Aesculap’s activL lumbar disc. “We’ll likely introduce it in the U.S. market early next year, and we’ve already started manufacturing on that product,” said Highridge Medical’s Chief Product Officer Ryan Watson.
He expects high demand for the new device. “We’re hearing from surgeons that more patients are requesting motion-preserving implants,” Watson said, “which makes sense because the average person intuitively recognizes that motion sounds better than fusion.”
Watson points out that surgical techniques for implanting motion-preserving devices can be more complex, often requiring specific approaches and specialized instrumentation.
“We develop everything surgeons need to get devices implanted into the patient. In many cases, that involves instruments specific to the implants that we’re developing,” he said. “We tell surgeons that we’re going to bring in instrument sets that allow them to start from the skin incision and get all the way to the right access path or corridor.”
Surgeon support and interest are pushing the motion preservation field forward. “Most surgeons who have tried motion preservation techniques prefer to use them,” Butters said. “Market forces and reimbursement challenges might push them to use fusion solutions, but they’ve seen some great clinical results with motion preservation.”
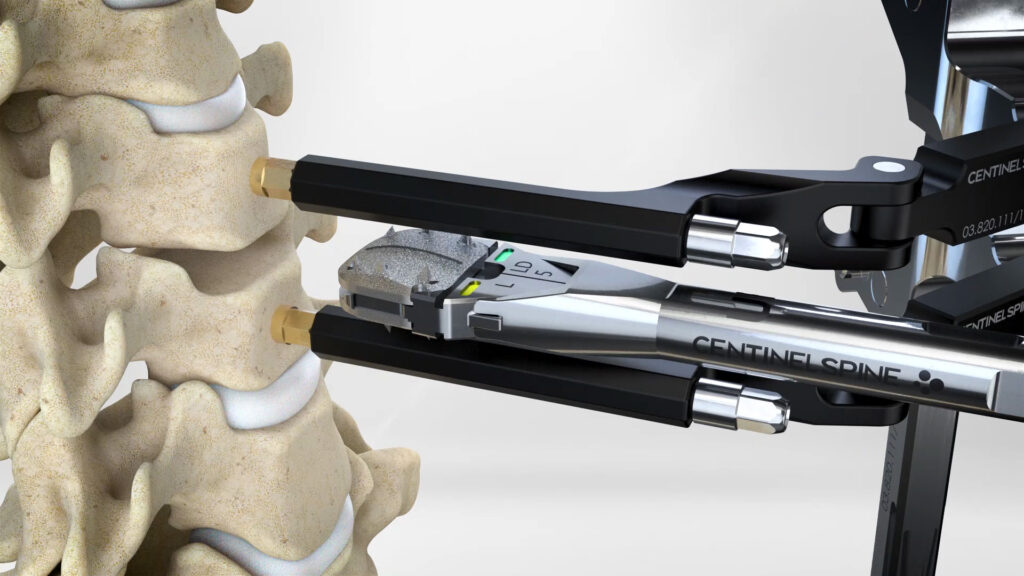
Centinel Spine’s prodisc technology features multiple motion-preserving anatomic solutions.
Design Improvements
Meeting device testing requirements is always a challenge, Watson said, adding that testing for a non-fusion device is already twice as intensive as for a fusion device.
“There are so many other types of loads and load patterns that these devices could face, so we end up spending a tremendous amount of time developing new testing methods,” he explained. “We work directly with FDA to come up with how to test motion-preserving implants. They’re much more complicated for us to study and understand.”
The Synergy Disc, Synergy’s flagship product, is commercially available in Australia, Europe, Canada, South Africa, Malaysia and New Zealand. The company has a CE mark and is in the process of getting full MDR certification. Butters said it’s the first cervical artificial disc to restore both alignment and balance while preserving natural physiological motion.
Last year, Synergy announced that it has completed patient enrollment in its U.S. two-level Investigational Device Exemption (IDE) clinical trial, which is being conducted on 200 patients at 24 clinical sites. The trial will evaluate the safety and effectiveness of the Synergy Disc compared to anterior cervical discectomy and fusion (ACDF) for the treatment of degenerative disc disease in patients who are symptomatic at two contiguous levels from C3 to C7.
Butters noted that cervical spine anatomy presents its own challenges when designing motion-preserving implants.
“You need a very small height to restore a vertebra’s natural movement and alignment, so both geometry and anatomy play into the process,” he said. “The loads, the amount of motion in each of the motion planes — and trying to replicate them during manufacturing — are significant challenges. Materials are also always key to making sure the spine’s function is restored as effectively as possible.”
Synergy has chosen to use time-tested orthopedic materials. “Our implant is a hard-on-soft bearing couple design, which is shown to work well in total joint replacements in knees and hips,” Butters said. “We chose titanium and ultra-high molecular weight polyethylene (UHMWPE) for our subtractive machining process.”
Butters thinks the potential of additive manufacturing applications in producing motion-preserving implants is currently unclear. “We require 100% dense, surface-finished parts, and I don’t believe anybody’s applying additive manufacturing to cervical discs,” he said, “but that’s not to say it won’t be applicable in the future.”
Although Synergy currently offers only a cervical implant, Butters sees good potential for the company in the lumbar space as well. In that capacity, he envisions a lot of demand for minimally invasive surgical tools and techniques.
“Designing conventional lumbar discs is challenging because they require an anterior approach that involves entering the peritoneal cavity, retracting organs and avoiding the great vessels,” he said. “Companies that develop devices that can be placed with a lateral or oblique approach would minimize the size of the wound and access port. I think those motion preservation solutions would do well in the future.”
Reducing Wear Resistance
All orthopedic companies work to prevent implant wear that can cause pain and loss of function over time. David Harding, Ph.D., Director of Materials Engineering at Dymicron, noted that even with the evolution of implant materials from stainless steel to cobalt chrome to polymers, the need to understand and control how an implant wears in the body continues.
“There is some good engineering happening throughout the industry,” Dr. Harding said. “But even so, the biggest concerns remain the wear particles and the negative impacts they cause.”
Dymicron’s Triadyme-C cervical artificial disc features bearing surfaces made from Adymite, the company’s proprietary polycrystalline diamond material. Adymite was developed specifically for high-stress, load-bearing environments and is engineered to dramatically reduce wear debris generation compared to conventional implant materials.
Triadyme-C’s Tri-Lobe design also mimics the natural kinematics and motion of a normal disc and enforces the proper translation and rotation of the spine during movement. It comprises the specialized polycrystalline diamond material that is harder and more wear-resistant than traditional implant materials.
Dymicron is currently marketing Triadyme-C outside the U.S. “We have our CE mark and are selling in Europe,” Dr. Harding said. “The CE mark unlocks much of the Latin American market, so we are expanding there, too.”
Triadyme-C recently received its IDE to begin a U.S. clinical trial. After enrolling patients across several leading U.S. spine centers, the company anticipates the first implantations will occur later this year.
Dymicron was founded by Bill Pope, Ph.D., a veteran of the oil drilling industry and Professor of Chemical Engineering at Brigham Young University. He was inspired to develop polycrystalline diamond when his friend needed joint revision surgery after only 10 years due to implant wear.
Dr. Pope realized that a potentially effective — albeit unusual material — was right at hand.
“Because we started with this amazing material, we didn’t need to figure out how to reduce implant wear,” Dr. Harding said. “Instead, we asked questions about how best to replicate the natural motion of the spine.”
Dymicron has faced manufacturing challenges due to the extreme pressures and temperatures required to produce polycrystalline diamond, but the company has developed specialized techniques to overcome these barriers.
“We’ve used a room-sized machine that produces one million PSI and goes up to 1,400 degrees Celsius,” Dr. Harding said. “That means we’re essentially replicating geologic conditions. The big breakthrough to make polycrystalline diamond possible is a centering alloy that allows carbon atoms to rearrange into the diamond structure.”
The result is a construct with exceptional hardness, low friction and long-term articulation performance, optimized for the demands of spinal motion preservation.
“Adymite allows our design team to focus on ways to replicate the natural biomechanics of the spine rather than just trying to reduce wear, which is a major challenge for other implant designers,” Dr. Harding said.
Forward Movement
Highridge is an established company in motion preservation, and Watson noted that extended 10-year follow-up clinical data from U.S. patients is now available for the Mobi-C, which was the first cervical disc FDA approved to treat more than one level of the cervical spine. FDA determined that Mobi-C was statistically superior to fusion at seven years for two-level cervical disc replacement.
“Now that we’re starting to see Mobi-C and some other devices with established clinical histories, patients and physicians are gaining confidence in the devices, and that is moving the market a bit,” Watson said.
As for what’s ahead in this space, Watson sees navigation and enabling technology aiding these surgeries.
“There’s an opportunity to understand and predict what the implant’s optimal height and size placement are and then help surgeons execute that plan,” he said. “Surgeons have already figured out how to determine the ideal implant placement, but with the help of artificial intelligence and enabling technology, I think we can get even more precise over time.”
When Dr. Harding considers major improvements in motion-preserving spine surgery, he singles out increasingly sophisticated cadaveric testing and in vivo CT scans.
“For a long time, the industry focused on range of motion in the spine, not the quality of motion after implantation. Being able to see how the spine is moving before and after surgery has been an important development,” he said. “A disc might be designed with a really large range of motion, but if the motion quality doesn’t match what the spine naturally wants to do, it won’t provide the necessary movement.”
DL
Darcy Lewis is a contributing writer.




