
Our latest review of newly marketable devices features solutions that build on the capabilities of established products or offer a new slant on current technology. Wenzel Spine wants to advance minimally invasive SI fusions with an FDA-cleared first, Arthrex’s high-resolution mobile imaging platform aims to improve pediatric arthroscopy and SurGenTec has expanded the indications of its synthetic bone graft beyond spinal fusion surgery.
Spine

Wenzel Spine | panaSIa Expandable Sacroiliac (SI) Fusion Implant, K250247
Submitted January 2025, granted July 2025
Primary predicate: Camber Spine Technologies, Camber Sacroiliac Fixation System, K233972
FYI: This marks the first-ever FDA clearance of an expandable SI fusion implant. The panaSIa implant is available now through a limited commercial release, with full commercial availability later this year.
Available in two sizes to suit patient anatomy, panaSIa SI Fusion is designed for minimally invasive dorsal placement using streamlined instrumentation. Its expandable architecture allows the implant to pierce the ilium and sacrum, stabilizing the SI joint and enabling post-expansion graft delivery without disengaging the instrumentation.
Sports Medicine
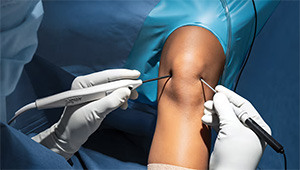
Arthrex | NanoScope, K243008 (expanded indications)
Submitted September 2024, granted January 2025
Primary predicate: Arthrex NanoScope System, K201134
FYI: This Traditional 510(k) premarket notification is submitted to expand indications for the Arthrex NanoScope System. Arthrex is also reporting software updates, device modifications and additional device models made since clearance in K201134.
The present clearance for the Arthrex NanoScope operative arthroscopy system addresses pediatric orthopedics (as well as laparoscopy).
NanoScope is a compact, high-resolution mobile imaging platform featuring the industry’s first high-definition, chip-on-tip camera — known as the NanoNeedle Scope — engineered specifically to meet the unique anatomical and procedural needs of pediatric patients.
The system can be used in common pediatric cases like general knee arthroscopy, meniscal treatments, anterior cruciate ligament reconstruction and general shoulder arthroscopy. The smaller scope is designed to minimize the potential risk of damaging anatomical structures upon entry along with neurovascular structures.
Orthobiologics
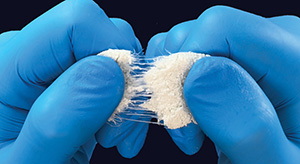
SurGenTec | OsteoFlo HydroFiber, K251720 (expanded indications)
FYI: This expansion of indications now includes use as a bone void filler for the treatment of tumors, cysts, trauma and osteomyelitis.
This latest addition complements SurGenTec’s previous clearance, which is recognized for its equivalence to autograft in spine surgery and is versatile enough for use at any spinal level, including for posterolateral fusions, disc spaces or interbody fusion cages.
OsteoFlo HydroFiber features Web Interlace Technology, which suspends particles within its fibers to effectively prevent graft migration, ensuring optimal cohesiveness and flowability. The intricate hydrophilic bonds and proprietary fibers allow for the wicking of saline, blood or bone marrow aspirate, creating a robust platform to support the healing process.
The product’s formulation offers optimal handling and resists migration under irrigation, permitting seamless application across various surgical settings, including spine, orthopedics and foot/ankle procedures. Additionally, it can be utilized with the GraftGun, SurGenTec’s flagship delivery device, to efficiently backfill interbody cages and tight spaces, serving as an alternative to traditional funnel methods.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




