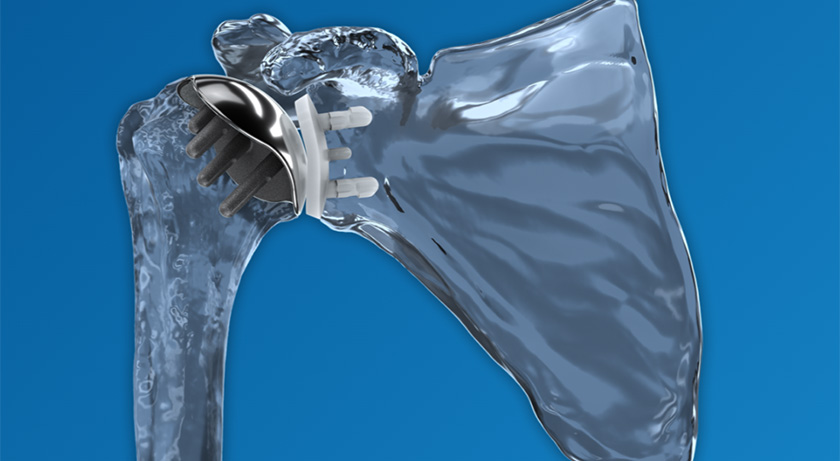
Catalyst OrthoScience commenced full commercial release of the Catalyst Fracture Shoulder System. FDA 510(k) marketing clearance for the system was secured in late 2024.
Designed to improve reverse shoulder arthroplasty for proximal humeral fractures, the system includes patented technology that helps reduce surgical complexity and drive clinical confidence.
The news comes on the heels of its recent announcement of five- to eight-year published midterm data supporting its Catalyst CSR Total Shoulder, the first product Catalyst launched to the market.
“At Catalyst, we’re not bound by convention – we’re defining what comes next,” said Carl O’Connell, president and chief executive officer of Catalyst. “Our technology is designed to solve real shoulder arthroplasty challenges, and our clinical outcomes prove the value of that approach. We’re committed to helping shoulder surgeons achieve better results with less complexity.”
Source: Catalyst OrthoScience Inc.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




