Trax Surgical received FDA 510(k) clearance to market the LINKT Compression Staple System. The staple system offers a range of nitinol staples for fracture repair, joint fusion and osteotomy procedures.
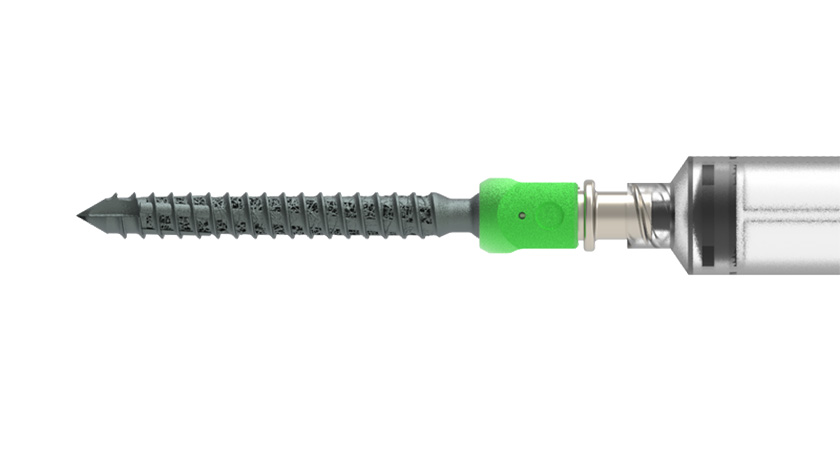
The LINKT Compression Staple features an adjustable inserter that allows the surgeon to open and close the staple’s legs for easy insertion. Multiple staples can be deployed with an appropriately sized inserter, which is also used to properly seat the implant or to remove and reposition it.
The company’s nitinol staples have a slightly curved bridge designed to provide more even compression across the fusion site and feature toothed legs for secure fixation. LINKT Staples are available in multiple bridge widths and leg lengths. The LINKT system comprises staples sold individually in sterile packaging and sterile deployment kits comprised of a drill, drill guide, locating pins and inserter.
Trax Surgical’s nitinol staple with inherent compressive properties creates a stable environment that promotes bone healing. “We are incredibly excited about this advancement,” said Shane Shankle, President of Trax Surgical. “The approval of our Nitinol Compression Staple enables us to better serve our customers and elevate the standard of care in extremity orthopedics. It reflects our dedication to innovation, quality, and the needs of both surgeons and patients.”
Source: Trax Surgical
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.