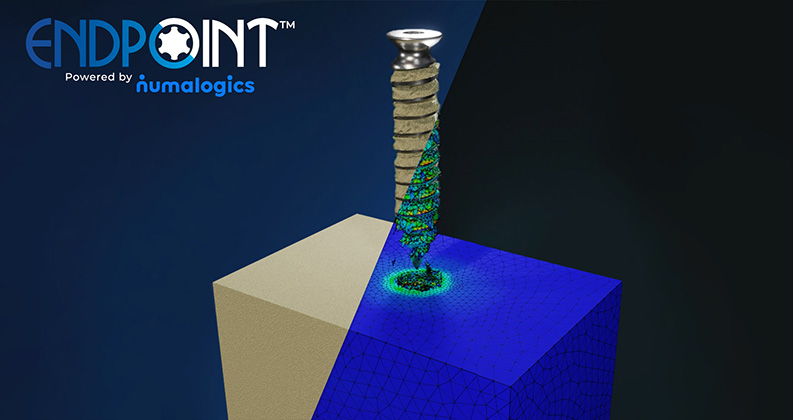
Sawbones’ and Numalogics’ jointly-developed Finite Element Analysis (FEA) model for orthopedic screw pullout testing received formal qualification from FDA through its Medical Device Development Tools (MDDT) program. This achievement marks the first-ever FDA qualification of a mechanical test for an orthopedic device, setting a new precedent for virtual testing in regulatory science.
The qualified tool simulates screw pullout behavior in accordance with ASTM F543, a widely accepted standard for evaluating the mechanical performance of orthopedic screws. Built on Sawbones’ industry-standard synthetic bone materials and powered by Numalogics’ advanced FEA modeling technology, the model enables accurate, reproducible virtual testing, reducing reliance on time-consuming and costly physical bench tests. This validated virtual method allows orthopedic device manufacturers to replace or supplement physical testing for both product design optimization and regulatory submissions.
“This landmark FDA qualification highlights and elevates the role of standardized bone simulation in medical device development and regulatory science,” said Amy Posch, M.S. Design Engineer-Biomechanical at Sawbones®, “Together with Numalogics, we’ve delivered a tool that is both scientifically rigorous and regulatory-ready.”
By qualifying this virtual test, FDA has recognized its ability to produce trustworthy and standardized evidence as a surrogate to physical testing using ASTM F543 screw pullout testing protocols and synthetic bone materials manufactured per ASTM F1839.
“This isn’t just a technical validation — it’s a shift in how the orthopedic industry can approach design and regulatory submissions,” said Eric Gaudreau, President of Numalogics. “With this FDA-qualified tool, companies can now leverage digital tools to reduce risk, accelerate development, and improve consistency.”
Source: Sawbones
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.