I enjoy finding less-familiar companies and sharing their products and technologies with you. Here are three companies that caught my attention this year. They offer solutions for sacroiliac joint fusion, foot & ankle repair and pre-surgical planning for shoulder replacement.
Spine

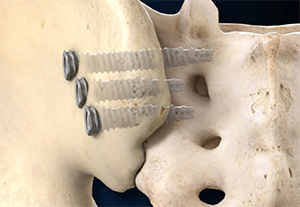
Promethean Restorative | DYNAMIS SI Screw System, K243565
Submitted November 2024, granted February 2025
Primary predicate: SI-BONE iFuse TORQ Implant System, K241574
FYI: Threaded, fenestrated, cannulated, 3D-printed titanium alloy implants and associated instruments indicated for sacroiliac joint fusion. DYNAVex negative bead porosity is designed to increase the screw body’s surface area by approximately 2x, doubling the potential for bone integration compared to a smooth screw body. DYNAMIS is the company’s first FDA 510(k)-cleared product. Promethean Restorative was founded in 2023 in Castle Rock, Colorado. Leadership brings experience from roles at Integra LifeSciences/Integra Orthobiologics and SeaSpine.
Trauma

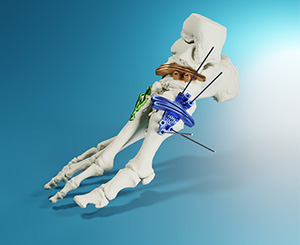
MedCAD | AccuStride System, K241811
Submitted June 2024, granted March 2025
Primary predicate: RedPoint Medical Better Bunion System, K220717
FYI: Coupled with the MedCAD’s proprietary software, the devices help foot and ankle surgeons correct multiple related pathologies in a single procedure. AccuStride is designed to improve proximal phalanx and metatarsal arthroplasty outcomes, and to provide custom solutions for Lapidus revisions and cavus foot corrections. Its components are based on UV-curable acrylate polymers or titanium alloy materials, and can be delivered in as few as five days after receipt of medical imaging and surgeon design approval. The system is intended for use in patients 12 years and older.
AccuStride marks the company’s first offerings for lower extremities. MedCAD also manufacturers patient-specific, custom-designed cranial and maxillofacial reconstruction implants. The company was established in 2009 with headquarters in Dallas, Texas.
Robotic/Digital

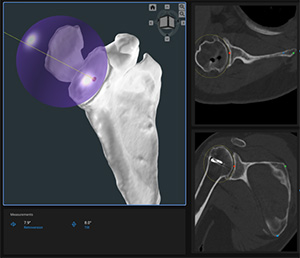
Precision AI | Precision AI Surgical Planning System (PAI-SPS), K243955
Submitted December 2024, granted January 2025
Primary predicate: Precision AI Surgical Planning System, K233992
FYI: The Precision AI Planning Software is intended to be used as a pre-surgical planner for simulation of surgical interventions for shoulder joint replacement. The software is used to assist in the positioning of shoulder components by creating a 3D bone construct of the joint that allows the surgeon to visualize, measure, reconstruct, annotate and edit presurgical plan data. The software is used to generate a surgery report and pre-surgical plan data file, which can be used as input data to design the Precision AI Shoulder Guide and Biomodels. The system is indicated for total and reverse shoulder replacement using implant systems from Enovis/Lima.
Precision AI, founded in 2019 by surgeons, mathematicians and engineers, is based in Queensland, Australia.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.