
It’s been a while since I was able to feature companies that gained their first FDA clearances to market orthopedic products in the U.S. These latest players introduced solutions to improve spine fusion surgery, enhance tissue fixation and fill bony voids/protect against infection.
Spine
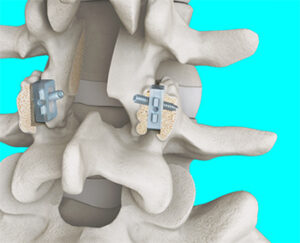
SC Medica | FFX Lumbar Facet Device, K232468
Submitted August 2023, granted May 2024
Primary predicate: Providence Medical Technology Facet Fixation System, Lumbar, K230840
FYI: The device is placed bilaterally through a posterior surgical approach and spans the facet interspace. FFX must be used with an FDA-cleared transfacet screw for use in the lumbar spine. The system is intended to provide temporary fixation and stabilization to the spine as an aid to lumbar fusion.
With seven years of follow-up in Europe, FFX has been successfully used in more than 10,000 implantations worldwide.
SC Medica claims to be the only spine company to clear a facet device from L3 to S1 levels (up to two levels) for placement with an intervertebral body fusion device or as an alternative to pedicle screw systems.
Sports Medicine
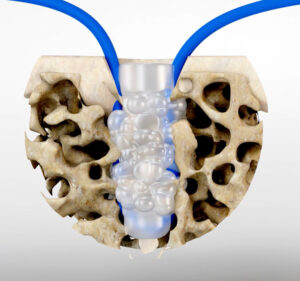
Surgical Fusion Technologies | SF Push-in Anchor, K240288
Submitted February 2024, granted April 2024
Primary predicate: SportWelding Fiji Anchor, K171228
FYI: The SF Push-in Anchor is intended for suture or tissue fixation in the foot, ankle, hand, wrist, elbow, hip, knee and shoulder. It is exclusively inserted with the SupraFuser Generator.
Employing SupraFusion technology creates ultrasonic vibrations that liquefy within a fraction of a second the outer surface of the polymeric suture anchor that contacts bone. The core of the suture anchor remains solid and stays cold. The liquefied polymer augments the cancellous bone at the interface to the implant, instead of violating it as a thread or barb of an anchor would do.
Orthobiologics
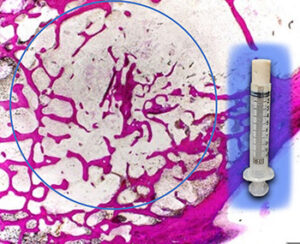
XeroThera | Xerosyn Calcium Bone Void Filler, K233259
Submitted September 2023, granted June 2024
Primary predicate: Baxter Healthcare Altapore, K192363
FYI: XeroSyn, a core platform of highly porous and nanostructured biomaterials, is an implant intended to fill bony voids or gaps of the skeletal system. It is used with autograft as a bone graft extender in the extremities and pelvis. XeroSyn resorbs and is replaced with bone during the healing process.
XeroThera’s technology platform was developed to support controlled, sustained release of antibiotics to prevent surgical site infections.
XeroThera is led by the co-founders of the orthobiologic company Orthovita, which was acquired by Stryker in 2011.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




