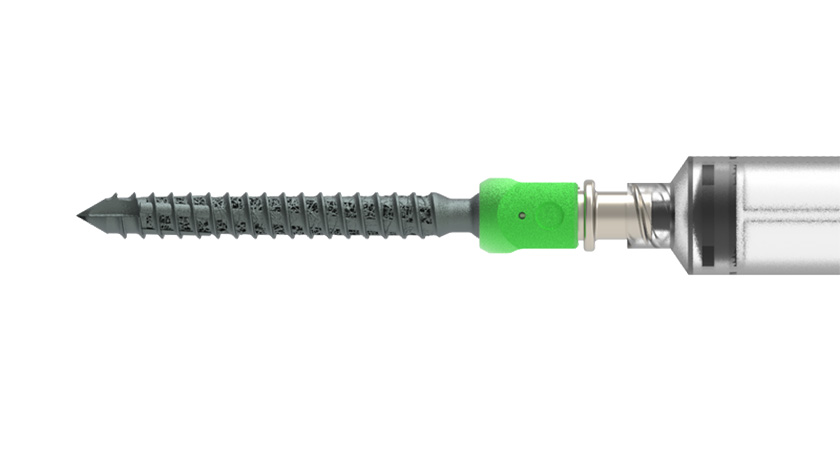
Smith+Nephew was granted FDA 510(k) clearance to market the CATALYSTEM Primary Hip System. The system is designed to address evolving demands of primary hip surgery, including the increased adoption of anterior approach procedures and the expanding role of Ambulatory Surgery Centers (ASCs).
Building on the heritage of Smith+Nephew’s stem designs, CATALYSTEM Primary Hip was developed using global data sets across femoral morphologies to help deliver a precision fit. Featuring a triple-taper stem design with uniform proximal loading, the reduced distal stem geometry and shorter lengths are ideal for anterior approach but suitable for all approaches.
CATALYSTEM also utilizes proprietary, patent-pending, ACCUBROACH Technology delivering reproducibility between broach and implant.
“Building on the strong clinical heritage of POLAR3◊, our CATALYSTEM Primary Hip System represents a significant milestone for Smith+Nephew’s hip business, complementing our current hip portfolio with a primary stem ideal for advanced anterior approaches, said Craig Gaffin, President Global Orthopaedics for Smith+Nephew. “Engineered for precision, confidence and surgical efficiencies, the launch of this new stem combined with our proprietary, market leading OXINIUM◊ Technology and integration with our robotics platform will help Smith+Nephew continue to enhance patient outcomes in hip surgery.”
Source: Smith+Nephew
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.