
This month’s recap offers joint replacement implants from BioPoly and Ortho Development, a spinal implant augmentation device from Woven Orthopedics and a hip fracture nail from OrthoXel.
Joint Replacement
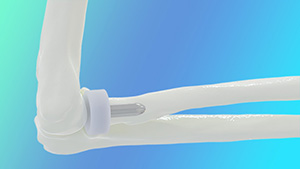
BioPoly Radial Head
BioPoly | Radial Head System, K233592
Submitted November 2023, granted March 2024
Primary predicate: Ignite Orthopedics, Revolution Radial Head, K183618
FYI: Hemiarthroplasty device designed to restore the articular surface and spacing of the proximal head of the radius in patients following fracture, resection and/or degenerative disability.
Reportedly the only synthetic cartilage-based radial head on the market. BioPoly material has repeatedly demonstrated that it does not damage the opposing cartilage surface like metal implants in multiple joint applications.
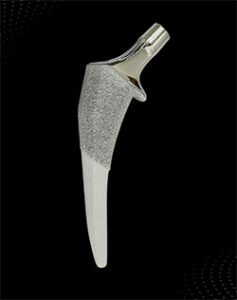
Ortho Development Trivicta Hip Stem
Ortho Development | Trivicta Hip, K233758
Submitted November 2023, granted March 2024
Primary predicate: DePuy Synthes Actis DuoFix Hip Prosthesis, K150862
FYI: Trivicta is a single-piece, tapered, collared and non-collared, hydroxyapatite and sintered bead commercially pure titanium coated femoral hip stem.
The triple-taper femoral stem supports an optimal fit within the canal for a diverse range of patient anatomies. Sintered beads provide initial stability and allow ingrowth for long-term stability, while hydroxyapatite promotes biological fixation.
A full U.S. launch will commence this year.
Spine

Woven Orthopedic Technologies Ogmend Implant Enhancement
Woven Orthopedic Technologies | Ogmend Implant Enhancement, K233223
Submitted September 2023, granted October 2023
Primary predicate: Woven Orthopedic Ogmend Implant Enhancement, K223075
FYI: The Ogmend implantable sleeve received its second clearance for the use of Ogmend Implant Enhancement in spine surgery, reportedly the first interface enhancement device that efficiently secures the entire screw/bone interface and provides multidimensional fixation enhancement.
Intended to augment pedicle screw fixation when a screw has lost purchase due to loosening, backout or breakage. The enhancement is to be used with rigid thoracolumbar pedicle screw systems where a screw is inserted posteriorly through a pedicle and into the vertebral body.
Trauma

OrthoXel Vertex Hip Fracture Nail
OrthoXel | Vertex Hip Fracture Nail, K233910
Submitted December 2023, granted April 2024
Primary predicate: Zimmer Biomet Affixus Hip Fracture Nail, K183162
FYI: Offers an alternative approach to fracture fixation, addressing challenges such as instability, which leads to high cutout rates, limited patient mobility post-surgery, persistent pain during recovery and nonunion complications.
Two proximal interdigitating screws produce enhanced screw fixation. The radially fluted nail stem is designed to ensure rotational and overall construct stability, particularly in the intertrochanteric and metaphyseal regions of the femur.
Three proximal construct options accommodate various fracture types, patient anatomies and intraoperative requirements. Surgeons can choose between two interdigitating lag screw configurations, with inferior or superior screw placement or, alternatively, a solid lag screw is available for a conventional proximal construct.
The implants and instruments feature radiographic guides and cues that allow for enhanced visualization during surgery.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




