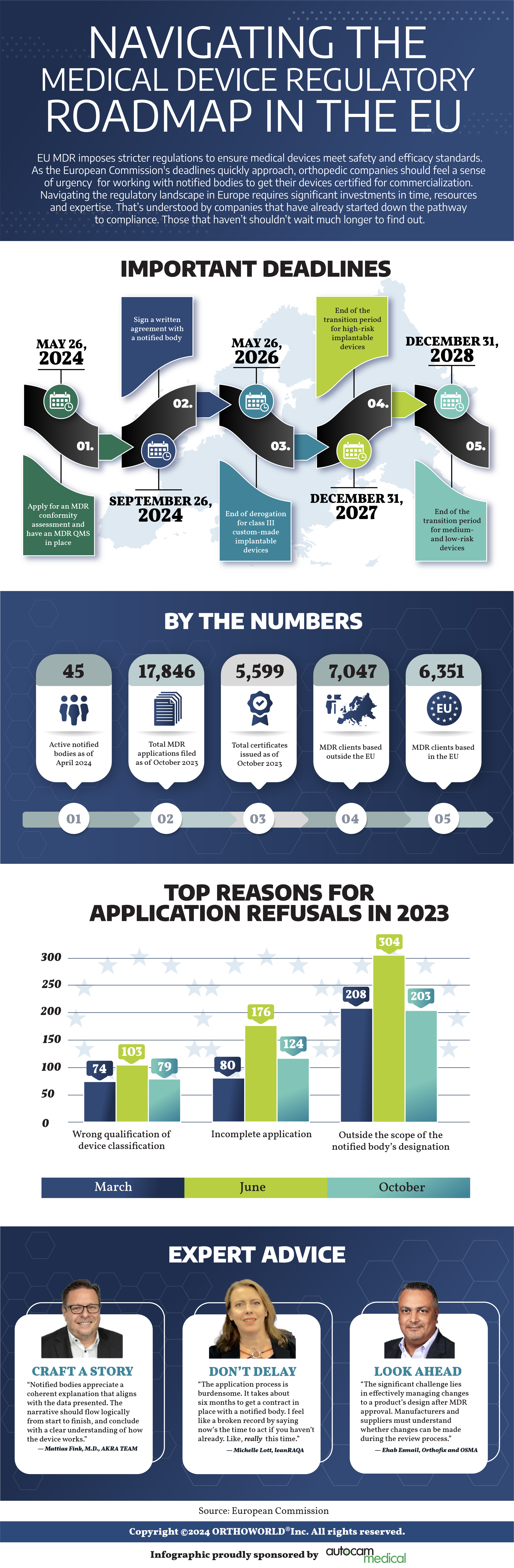
The European Commission has postponed the Medical Device Regulation (MDR) transition deadline twice, extending the time orthopedic companies have to certify medical devices for commercialization in European markets.
Notably, the transition deadline of May 26, 2024 has been extended to December 31, 2027 for high-risk devices and December 31, 2028 for medium- and low-risk devices. That doesn’t mean orthopedic companies should alter their compliance strategies.
Companies that are well into the review process with a Notified Body should continue to move forward with their efforts. Those that haven’t should begin working with a notified body as soon as possible.
Keep in mind that applying for MDR certification requires a team of multidisciplinary experts to tackle the clinical, technical and regulatory requirements. Notified bodies should do their part by providing device manufacturers with regulatory guidance and technical information on how to apply for conformity assessments.
Many orthopedic companies are navigating the learning curve of MDR compliance and must continue to stay current on the regulatory roadmap and elicit the help of experts to reach the finish line.
Infographic proudly sponsored by Autocam Medical.