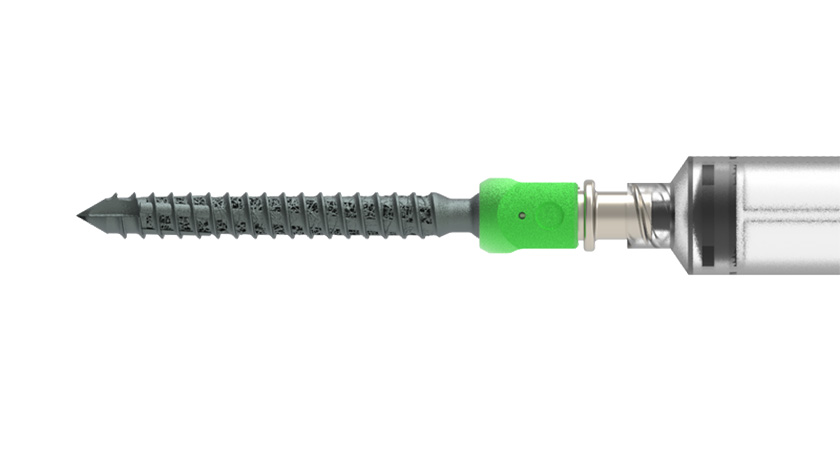
Carlsmed announced a $52.5 million Series C funding round co-led by B Capital and U.S. Venture Partners. Proceeds support accelerated commercialization of the aprevo personalized spine surgery platform for lumbar fusion procedures and the development of aprevo for cervical fusions, which will launch in 2025.
Carlsmed received Breakthrough Device designation by FDA for aprevo lumbar and cervical patient-specific interbody fusion devices. Carlsmed implantable devices and software platforms are FDA-cleared for lumbar spine fusion, including anterior, lateral and transforaminal approaches.
“We started Carlsmed to improve patient outcomes through personalized surgery, and recent clinical publications indicate that aprevo patient specific implants are achieving this goal,” stated Mike Cordonnier, CEO of Carlsmed. “Our AI-enabled technology platform and innovative business model allow us to scale production rapidly to meet growing demand and empower patients.”
Source: Carlsmed
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.