
This month’s review of recent 510(k) clearances is a catch-all of innovative solutions, from better kinematic alignment of knee ligaments during joint replacement surgery to an artificial intelligence (AI)-based tool that improves the treatment of scoliosis. A 3D-printed sacroiliac fixation system, a versatile distal radius plating system and a new graft for interbody spinal fusions round out new entries in the market.
Joint Replacement
TJC Life | PNK (Preservation of Normal Knee Kinematics) Total Knee, K231975
Submitted July 2023, granted November 2023
Primary predicate: RootLoc Acculoc Total Knee, K170753
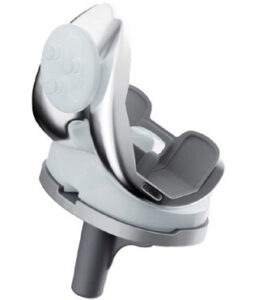
TJC Life | PNK Total Knee
FYI: PNK Total Knee is indicated for cemented primary and revision procedures. The fixed bearing implant includes a posterior stabilized design and cruciate retained configurations. The implant’s design is customized for Koreans of smaller stature with a sedentary lifestyle and is capable of high flexion of 150 degrees.
TCJ Life also developed an augmented reality system that integrates computed tomography and magnetic resonance imaging of patients, as well as KNEESIGN virtual reality/simulated surgery software for educational purposes.
PNK is the company’s first orthopedic-related FDA 510(k) clearance.
Spine
Xēnix Medical | SOLACE Sacroiliac Fixation System, K233241
Submitted September 2023, granted October 2023
Primary predicate: Xēnix Medical Sacroiliac Fixation, K231829

Xēnix Medical | SOLACE Sacroiliac Fixation
FYI: The SOLACE Sacroiliac Fixation System comprises 3D-printed, threaded implants ranging from 10.5mm to 12.0mm in diameter and 30mm to 115mm in length. Implants feature Xēnix Medical’s NANOACTIV surface technology and incorporate helical autograft harvesting flutes and porous channels for bony ingrowth running the length of the device.
The NANOACTIV micro- and nano-roughened surface is designed to improve fixation to adjacent bone. These features elicit an endogenous cellular and biochemical response as represented by the differentiation of human mesenchymal stem cells through the osteogenic lineage and production of a mineralized matrix in vitro.
SOLACE is intended for sacroiliac fusion; augmentation, immobilization and stabilization of the sacroiliac joint in skeletally mature patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion. It’s also intended for fixation of acute, non-acute and non-traumatic fractures involving the sacroiliac joint.
The system is cleared for navigation of SOLACE implants and instrumentation using the Medtronic StealthStation system and NavLock trackers to assist surgeons in locating anatomical structures in open or minimally invasive procedures.
Trauma
Tyber Medical | Distal Radius Plating System, K232693
Submitted September 2023, granted December 2023
Primary predicate: Tyber Medical Anatomical Plating, K203871
Tyber Medical | Distal Radius Plating
FYI: The Distal Radius Plating System maintains stability and orientation in the anteroposterior plane by utilizing a dynamic grouping of plate length options offered in titanium or stainless steel. The system’s design covers a range of fractures, fusions, non-unions, malunions and osteotomies of the radius, ulna and hand.
An enhanced ergonomic design reduces flexor tendon irritation, a common postoperative concern.
Orthobiologics
Kuros Biosciences | MagnetOs Flex Matrix, K233245
Submitted September 2023, granted November 2023
Primary predicate: NuVasive AttraX Putty, K203714

Kuros Biosciences | MagnetOs Flex Matrix
FYI: MagnetOs Flex Matrix has already been cleared by FDA for use in posterolateral fusion. Now it has received clearance for interbody fusions performed in the cervical, thoracic and lumbar spine — the first Kuros product to achieve the designation. Due to its fibrillar and flexible structure, this open matrix graft promotes bone growth even in soft tissue by optimizing the effect of Kuros’ NeedleGrip surface technology.
Robotics/Tech
NSite Medical | AI-Based Scoliosis Scanning Application, K230463
Submitted February 2023, granted November 2023
Primary predicate: Quantec Spinal Measurement System, K923792
FYI: NSite Medical’s AI-based application utilizes advanced algorithms to analyze spinal curvature with high precision, facilitating early diagnosis and more effective treatment strategies for patients with scoliosis.
NSite is a portfolio company of the venture capital firm cultivate(MD). ProVoyance, another portfolio company, played a pivotal role in the development of this application. ProVoyance’s expertise in medical software development helped to advance the application’s market introduction and secure its FDA clearance.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




