
All three companies profiled this month are hitting the orthopedic market with their first FDA-cleared products. Their products address diverse patient needs, including partial knee resurfacing, sacroiliac (SI) joint fixation and fracture repair in the extremities.
Joint Replacement
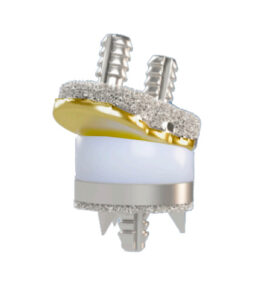

Submitted May 2022, granted March 2023
Primary predicate: Arthrosurface Unicompartmental Knee Resurfacing Prosthesis (UniCAP), K050373
FYI: The OvertureTi Knee Resurfacing’s femoral and tibial baseplates are 3Dprinted in porous titanium and the tibial components use overmolded polyethylene.
- The system is intended for cemented use in partial knee replacement when patients experience compartmental degenerative disease, post-traumatic degenerative disease, tibial condyle or plateau fractures, deformity or have undergone previous arthroplasty.
- Overture refers to the technique of replacing only the affected areas and preserving healthy tissue as Focalplasty.
- The company was founded in 2020 and is headquartered in Irvine, California. The leadership team brings experience from roles at Acumed, Carlsmed, Enovis, ExsoMed, Insight Medical Systems, Integra LifeSciences, LifeModeler, OrthAlign, Orthohelix, Smith & Nephew, Stryker Orthopaedics and Zimmer.
Spine
Xenix Medical | Sacroiliac Fixation System, K231829
Submitted June 2023, granted August 2023
Primary predicate: SI-Bone iFuse TORQ Implant System, K222605
FYI: Xenix’s system is indicated for treatment of SI joint dysfunction, including degenerative sacroiliitis, joint disruptions and fractures.
- The company has developed neoWave technology, a 3D-printed homogenous waveform matrix designed to enhance load dispersion while reducing device stiffness and the potential for subsidence.
- Xenix Medical, formerly HT Medical, was founded in 2020 and is based in Orlando, Florida. Leadership held previous roles at Alphatec Spine, Engage Surgical, Integra LifeSciences, LifeLink Tissue Bank, Optimotion Implants, Parcus Medical, Sanara MedTech, SeaSpine and Sendia Biologics.
Trauma
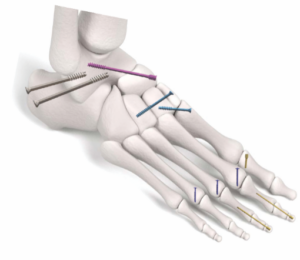

Submitted April 2023, granted September 2023
Primary predicate: Globus Medical Captivate Compression Screws, K222409
FYI: Trax’s Accelerate screws come in headed and headless formats and are intended to treat fractures and reconstruction of small hand and feet bones.
- Launched by Primo Medical this year, Trax Surgical is located in Stoughton, Massachusetts. The compression screws were originally developed by Primo, a company with over 40 years of experience in the design, development and manufacturing of medical devices and implants. Arthrosurface, acquired by Anika, was incubated by Primo.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




