
This month’s roundup features FDA 510(k) clearances for versatile and stable spine fusions, fracture fixation in pediatric patients and new additions to Stryker’s trauma plate portfolio. It also includes a suturing device for hip repair and the first clearance for OnPoint Surgical, a new player in the field of augmented reality.
Spine
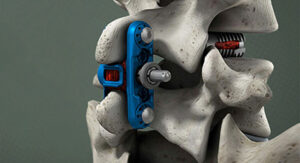

Wenzel Spine | primaLOK SP, K231807
Submitted June 2023, granted August 2023
Primary predicate: LESpine Innovations Inspan ScrewLES Fusion, K213266
FYI: This clearance addresses new clinical indications that allow primaLOK SP to be used at multiple vertebral levels and to treat patients with lumbar spinal stenosis.
primaLOK SP features include:
- A locking mechanism that ensures secure stabilization, reducing the risk of migration and providing stability during the critical fusion process.
- Flexibility to accommodate various anatomies and offer surgeons the ability to tailor each procedure to individual patient needs.
- A minimally invasive approach to minimize tissue disruption, potentially leading to reduced postoperative pain, shorter hospital stays and quicker patient recovery.
- Enhanced radiographic visibility, allowing surgeons to assess implant placement and fusion progress.
- Wenzel Spine acquired primaLOK systems from OsteoMed in 2017.
Trauma
OrthoPediatrics | Pediatric Nailing Platform (PNP) Tibia, K231266
Submitted May 2023, granted August 2023
Primary predicate: Pega Medical Simple Locking Intramedullary (SLIM) System, K192710
FYI: PNP Tibia represents another pediatric-focused solution for treating patients with fractures and deformities in the lower extremities. Launch is slated for 3Q23.
The system features rigid cannulated nails, ranging in diameter from 7mm to 12mm, and includes specialized instrumentation to facilitate multiple surgical techniques.
Like other OrthoPediatrics products, this system was designed to fit children’s anatomy and accommodate growing bones.
Stryker | Pangea Trauma Plating Systems, K231262 and K231257
Submitted May 2023, granted August 2023
Primary predicates: Stryker AxSOS 3 Ti System, K200398; Stryker PeriPRO Femur and Variable Angle Fixation, K222381
FYI: Two separate clearances address systems for femur, fibula, tibia, humerus and utility applications. The latter is generally indicated for internal fixation and stabilization of bone fractures, osteotomies and arthrodesis in normal and osteopenic bone.
Pangea plates were designed to enhance plate fit and screw placement, and offer anatomically contoured implants for patient populations with a wide variety of fracture patterns.
Streamlined instrumentation and implant trays include 20 anatomic plates and 13 utility plates in one platform.
Sports Medicine


Submitted September 2021, granted June 2023
Primary predicate: Arthrex Suture Grafting Kit, K041553
FYI: This subject clearance addresses application in hip labral reconstruction and augmentation.
AceConnex is intended for use as a component in soft tissue surgical procedures where constructs — including those with allograft tissue — are used for reconstruction, replacement or augmentation of the labrum.
The ready-to-use, sterile device will be available in multiple pre-sutured sizes, with trimmable regions that allow for allograft adjustments to match patients’ anatomy.
AceConnex is manufactured to ensure consistency and minimize variability compared to allografts that are manually sutured preoperatively.
Robotics/Digital


OnPoint Surgical | Augmented Reality (AR) Spine System, K231284
Submitted May 2023, granted September 2023
FYI: The OnPoint AR Spine System is an open platform that’s compatible with the implants of major manufacturers. It offers a cost-effective and highly accurate alternative to current robotic systems. Surgeons who use the system don’t have to change their surgical techniques or routines.
The system is readily implemented in hospital settings and its small footprint makes it well suited for ASCs. The head-mounted display is four to ten times lighter and has up to four times greater resolution than existing AR spinal systems.
OnPoint Surgical and affiliates OnPoint Medical, OnPoint Knee, OnPoint Hip and OnPoint Sports are developing AR guidance for multiple surgical applications. The OnPoint AIm-AR technology platform is being developed further for all spinal, neurosurgical, orthopedic, arthroscopic, robotic and other surgical procedures.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




