
This month’s roundup of recent FDA 510(k) clearances includes a new shoulder replacement, a company’s first sacroiliac joint implant, a novel device for rotator cuff repair and a spine navigation platform that enhances the placement of pedicle screws. These new technologies come from established companies and newer entries to the market.
Joint Replacement
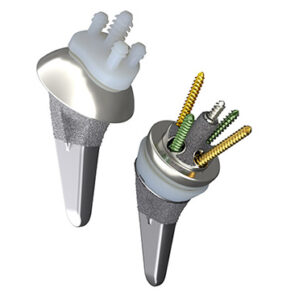
Smith+Nephew | AETOS Shoulder Replacement
Smith+Nephew | AETOS Shoulder System, K230572
Submitted March 2023, granted June 2023
Primary predicate: Smith+Nephew AETOS Shoulder, K220847
FYI: The AETOS portfolio, which is indicated for anatomic and reverse total shoulder arthroplasty, supports intraoperative flexibility. With fewer steps for conversion and fewer instruments for primary anatomic and reverse replacements, the system is designed to simplify the surgical workflow.
In anatomic applications, the system’s stem and head may be used by themselves as a hemiarthroplasty if the natural glenoid provides a sufficient bearing surface, or in conjunction with the glenoid as a total replacement.
Spine
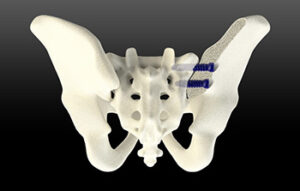
SurGenTec | TiLink-L Sacroiliac Joint Fusion System
SurGenTec | TiLink-L Sacroiliac Joint Fusion System, K230446
Submitted February 2023, granted June 2023
Primary predicate: Globus Medical SI-LOK Sacroiliac Joint Fixation, K112028
FYI: SurGenTec’s first implant in its sacroiliac family of products can be implanted from a lateral or posterior/oblique approach and its design supports compression across the joint.
The system features a helical self-harvesting channel to capture the patient’s own bone. Graft windows are strategically placed between threads to allow for potential fusion through the screw and enhanced stability.
The implant is equipped with SurGenTec’s proprietary Nanotex surface technology to increase bony integration.
Sports Medicine

Inovedis | SINEFIX Implant System
Inovedis (submitted by BAAT Medical Products) | SINEFIX Rotator Cuff Repair, K220966
Submitted April 2022, granted March 2023
Primary predicate: CoNextions Medical Coronet Soft Tissue Fixation System, K200028
FYI: SINEFIX is a PEEK implant that consists of a baseplate with a lateral and a medial anchor. The baseplate is placed over the torn rotator cuff tendon and attached to the humerus with the anchors. The medial anchor goes through the tendon and the lateral anchor goes directly into the bone.
The implant reattaches the rotator cuff tendon to bone with a simplified surgical technique that has been optimized for minimally invasive surgery. The technique aims to significantly reduce operative time and cost of the procedure.
SINEFIX creates a flat and even contact of tendon and bone. It distributes the shear stress uniformly and does not cause punctual pressure peaks, while also maintaining blood circulation and supporting the healing process.
Robotic/Digital
PathKeeper Surgical | PathKeeper Spine Navigation System, K222355
Submitted August 2022, granted March 2023
Primary predicate: 7D Surgical Envision 3D Image Guidance System, K162375
FYI: PathKeeper’s surgery planning and navigation software and 3D optical camera help surgeons precisely locate anatomical structures and place pedicle screws in the thoraco-lumbo-sacral region during posterior lumbar interbody fusion.
PathKeeper is designed to offer active, real-time and independent tracking of patient anatomy and surgical instruments It also allows surgeons to place screws within a millimeter of the intended target, contributes to more efficient surgical workflows, eliminates radiation exposure during the surgical procedure and is available at an economical price point for hospitals and ASCs.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




