
The latest FDA 510(k) clearances feature solutions from Formus Labs, Proprio, THINK Surgical and SMAIO with the potential to improve hip replacement and spine surgery outcomes.
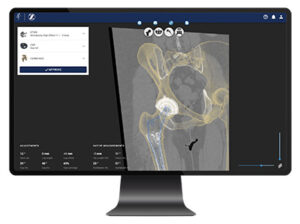
Formus Hip
Formus Labs | Formus Hip, K213272
Submitted September 2021, granted March 2023
Primary predicate: Peek Health Peekmed, K182464
FYI: Described as the first automated radiological image processing software for hip replacement planning. The technology combines artificial intelligence with computational biomechanics to calculate a patient’s implant fit and deliver digestible, actionable and fully interactive 3D surgical plans in under an hour.
This capability provides surgeons with personally curated surgical plans before patients enter the O.R.
In 2022, Formus Labs partnered with Zimmer Biomet to co-develop and commercialize Formus Hip in Australia and New Zealand and investigate international markets for global expansion.
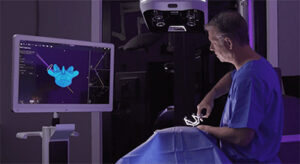
Proprio | Paradigm Surgical Navigation Platform
Proprio | Paradigm Surgical Navigation Platform, K222291
Submitted August 2022, granted April 2023
Primary predicate: 7D Surgical Envision 3D Image Guidance System, K162375
FYI: Designed to offer an alternative to traditional navigation technologies that distract the surgeon’s attention from the patient and disrupt surgical workflows.
Paradigm is reportedly the first spine navigation platform to use light field technology to create a real-time 3D view of anatomy and the surgical scene. The system uses an advanced sensor suite to capture high-definition multimodal intraoperative images and fuses that information with preoperative scans. Proprio refers to this data fusion capability as Volumetric Intelligence.
Paradigm can collect a high volume of clinical information with approximately 250GB of data captured per hour. Such data drives Proprio’s product development process and informs advanced models for future applications.
SMAIO (Software, Machines and Adaptative Implants in Orthopaedics) | KBA3D, K223841
Submitted December 2022, granted May 2023
Primary predicate: SMAIO KEOPS Balance Analyzer 3D, K213975
FYI: SMAIO received clearance for a customized surgery planning software that was co-developed with NuVasive. The clearance is an important milestone in the partnership and licensing agreement and triggers a $3 million payment by NuVasive to SMAIO. An additional milestone payment will be triggered after further integration of SMAIO’s Balance Analyzer 3D spinal realignment planning software and additional technical capabilities into the NuVasive platform.

THINK Surgical | TMINI Miniature Robotic System
THINK Surgical | TMINI Miniature Robotic System, K230202
Submitted January 2023, granted April 2023
Primary predicate: Orthosoft (Zimmer CAS) ROSA Knee System, K182964
FYI: TMINI replaces many of the instruments currently used for knee replacement surgery. The system includes a wireless robotic handpiece that assists surgeons in performing total knee replacement.
Following a CT-based three-dimensional surgical plan, the TMINI robotic handpiece automatically compensates for surgeon hand movement to locate bone pins along precisely defined planes. Cutting guides are then connected to the bone pins for accurate bone resection.
THINK Surgical is committed to an open implant library and will continue to add new implant options to the platform over time. This open implant approach, combined with the ease of use of the TMINI system, should appeal to a broad customer base who may have been resistant to robotics.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




