This month’s roundup of recent FDA 510(k) clearances features new products in spine and trauma, the second and third largest subsegments in orthopedics. The spine solutions improve bone growth and pedicle screw fixation. Entries in the trauma space focus on fixing fractures in the femur and treating trauma of the foot, ankle and wrist.
Spine

Curiteva Inspire Cervical Interbody
Curiteva | Inspire Porous PEEK Cervical Interbody System, K213030
Submitted September 2021, granted February 2023
Primary predicate: Curiteva Cervical Interbody Fusion System, K181261
FYI: Curiteva said that this is the first FDA 510(k)-cleared 3D-printed PEEK implant. It combines HAFUSE nanotechnology surface treatment and a novel porous PEEK structure to create a hydrophilic, bioactive environment for cell attachment, proliferation and healing. The company plans a commercial launch in key academic centers across the U.S.
Curiteva’s 3D printing process and its HAFUSE coating maintains the benefits and overcomes the limitations of the PEEK. Printing an engineered lattice structure with fully interconnected porosity provides superior mechanical strength and achieves a modulus of elasticity that closely matches human cancellous bone, according to the company.
Woven Orthopedics Ogmend Implant Enhancement System
Woven Orthopedic Technologies | Ogmend Implant Enhancement System, K223075
Submitted September 2022, granted February 2023
Primary predicate: Woven Orthopedic Ogmend Implant Enhancement System, De Novo DEN180065
FYI: This clearance covers the augmentation of pedicle screw fixation during spine surgery when a screw loses grip due to loosening, back-out or breakage. In 2020, Ogmend received FDA De Novo clearance for use in long bone trauma surgery.
Ogmend is an implantable sleeve designed to aid screw fixation in challenging scenarios. It is made of polyethylene terephthalate (PET) monofilament fibers and provides a helically braided structure that is captured when a screw is advanced during placement. When it is difficult to achieve secure fixation between screws and bone, the Ogmend sleeve offers an off-the-shelf solution. It can be deployed in under two minutes and is used with a wide range of screw systems from a variety of suppliers.
Ogmend will be available in the U.S. through a staged, regional release.
Trauma
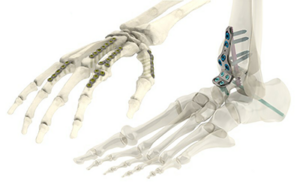
Tyber Medical Anatomic Plating
Tyber Medical | Anatomical Plating System, K222465
Submitted August 2022, granted March 2023
FYI: With this new clearance, Tyber Medical’s Anatomical Plating System has 391 additional plate configurations, including 100 new plates targeting foot, ankle and wrist indications. FDA deemed the Anatomical Plating System safe to carry MR Conditional labeling, addressing its use in the MRI environment under certain tested conditions.
The Anatomical Plating System is indicated to treat a comprehensive range of deformity, trauma and degenerative conditions of the wrist, foot, ankle and long bones.
Tyber Medical officially launched its Anatomical Plating System in 2020, when the product line received its initial 510(k) clearance. The latest version of the system includes new plate sizes, instrumentation and configurations for short bones, long bones and ankle fracture/fusion.
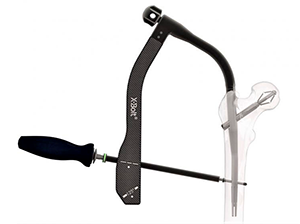
X-Bolt Pro-X1 Trochanteric Nail
X-Bolt | Pro-X1 Trochanteric Nail, K221621
Submitted June 2022, granted March 2023
FYI: The Pro-X1 Trochanteric Nail features a titanium construct with a reversibly expanding bolt instead of a typical lag screw. The expanding bolt enables stronger anchorage and rotational stability of the femoral head. The system’s instrumentation includes the patented Metro Jig, a curved jig with flexi-drive coupling, to streamline the operative technique. It is intended for fracture fixation in the femur in adults with osteopenia or osteoporosis.
Pro-X1 will be X-Bolt’s flagship launch in the U.S. It builds off the company’s foundational expanding bolt design, which was clinically proven to have an 0.8% cut-out rate in the largest-ever hip fracture randomized controlled trial.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




