Share on:
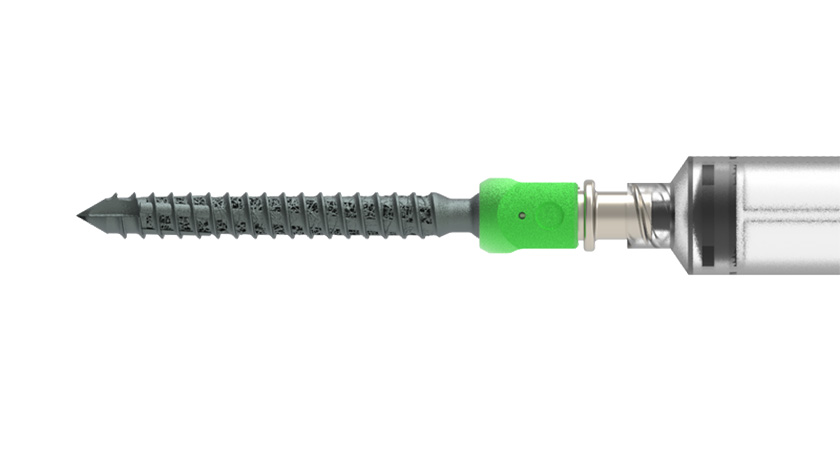
Eminent Spine received FDA 510(k) clearance to market its 3D-printed titanium Cervical Interbody Fusion Device (IBFD).
The Cervical IBFD system was designed with the following features:
- Aggressive teeth to resist migration
- Tapered nose for ease of insertion
- Lordosis allows for ease of insertion and self-distraction
- Large central opening for maximum bone graft material
- Universal inserter for all implant profiles.
Eminent Spine offers PEEK, machined titanium and 3D-printed titanium implants. The implants are offered non-sterile.
Source: Eminent Spine, LLC
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.
RELATED ARTICLES
Zimmer Biomet Gains PMDA Approval of Iodine-Treated Total Hip
Sep 26 2025 , Julie A. Vetalice