Our recap highlights companies that recently announced FDA clearance of devices in the joint replacement, spine, trauma, sports medicine and digital market segments. The solutions focus on improving the accuracy and effectiveness of spine fusions, enhancing bone resurfacing and fixation and optimizing the outcomes of ACL reconstruction in pediatric patients.
Joint Replacement
BioPoly Lesser Toe Implant
BioPoly | BioPoly Lesser Toe, K222964
Submitted September 2022, granted November 2022
Primary predicate: Vilex Met-Head, Resurfacing Hemi-Arthroplasty Implant and Cannulated Hemi Implants, K070052, K190543
About the product: The implant replaces one side of a toe joint with a cartilage-friendly surface and robust bone fixation to maintain motion at the joint while keeping healthy cartilage intact. BioPoly’s biomaterial is a combination of polyethylene and hyaluronic acid. This proprietary blend results in a strong, hydrophilic material that behaves like synthetic cartilage with the ability to articulate with native cartilage while minimizing damage to native tissue.
Spine

SI-BONE iFuse Bedrock Granite Implant
SI-BONE | iFuse Bedrock Granite Implant, K222774
Submitted September 2022, granted December 2022
Primary predicate: SI-BONE iFuse Bedrock Granite Implant, K220195
About the product: SI-BONE received additional clearance for its iFuse Bedrock Granite Implant, which is typically used to immobilize and fuse the sacroiliac (SI) joint and provide support at the base of spine fusion constructs. The device’s initial clearance included an indication for use with a single manufacturer’s pedicle screw system. The expanded indications include use with a wide range of rods that are commonly used in multilevel spine fusion. Surgeons can now use their preferred techniques and implant systems with iFuse Bedrock Granite as the foundation for their construct.
Trauma

Tyber Medical NiTy One Shot Staple Fixation
Tyber Medical | NiTy+ One-Shot Staple Fixation, K221947
Submitted July 2022, granted October 2022
Primary predicate: DePuy Synthes/Biomedical Enterprises Speed, Speed Shift, Speed Titanium and Elite Nitinol Systems, K142292
About the product: Reportedly the first tool of its kind in the bone fixation market to feature a single-pass insertion instrument. The product is suitable for fracture repair, osteotomy fixation and joint arthrodesis. It combines pre-loaded staples and a tamping mechanism into a single insertion tool, eliminating the need for a second instrument to fully seat bone fixation staples. The tool can also reattach to the staple for enhanced control.
Sports Medicine
Arthrex ACL TightRope
Arthrex | Arthrex ACL TightRope Implant, K221128
Submitted April 2022, granted October 2022
Primary predicate: ACL TightRope, K112990;
About the product: Arthrex’s ACL TightRope implant received clearance for pediatric indications, reportedly making it the only fixation device designed for treating anterior cruciate ligament (ACL) injuries in children. The ACL TightRope implant portfolio includes the ACL TightRope II, the TightRope attachable button system, the FiberTag TightRope and the ACL Repair TightRope with FiberRing sutures. Arthrex worked with surgeons at the Hospital for Special Surgery to develop all-epiphyseal and transphyseal techniques and instrumentation for ACL surgery in skeletally immature patients. The all-epiphyseal technique involves the use of pediatric-specific instrumentation guides to drill sockets for the reconstructed ACL while avoiding the growth plate, thereby reducing the potential for growth disturbance.
Robotic/Digital
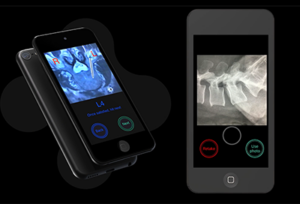
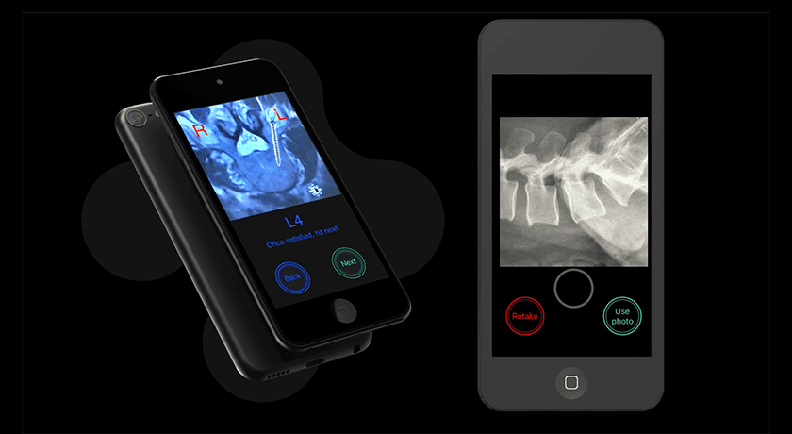
Circinus Medical Bolt Navigation Technology
Circinus Medical Technology | Bolt Navigation System, K213768
Submitted December 2021, granted December 2022
Primary predicate: Track X Technology TrackX Device, K200360
About the product: Instrument tracking technology that assists in the accurate placement of pedicle screws when used in conjunction with intraoperative fluoroscopy. The platform utilizes an iOS device with a high-powered processor, familiar user interface and gyroscope-on-chip technology. It features a near-zero footprint, an instrument-agnostic design, the ability to utilize standard imaging and a subscription-based business model.
JAV
Julie A. Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.




