
Cartilage damage and degeneration is a significant clinical issue that lacks effective methods for repair and replacement. Orthopedic companies like CartiHeal (now Bioventus) and Vericel have commercialized cartilage replacement products in recent years, but a substantial market opportunity remains for players in the sports medicine space. Nanochon is one startup that seeks to fill the market’s void with its 3D-printed biomaterial.
The company describes its Chondrograft as “a sturdy medical advancement that is one part orthopedic load-bearing implant, one part tissue growth scaffold and completely revolutionary.” The company is highly focused on developing an off-the-shelf device that easily integrates into a surgeon’s workflow. Between the technology and surgical technique, Nanochon aims to commercialize an implant that provides a longer-lasting, more affordable solution to joint disease and injury for younger, more active patients.
“Ultimately, we’re looking at being able to treat half a million patients a year,” said Benjamin Holmes, Ph.D., Nanochon’s Co-founder and CEO. “There are 700,000 knee arthroscopies performed each year and, excluding microfracture procedures, about 25,000 corrective procedures involve an implant. Our technology is designed to deliver better clinical outcomes. Having a truly commoditized implant, both in terms of ease of use and cost, will allow that six-figure group of patients to benefit.”
Nanochon’s Chondrograft is on an FDA premarket approval pathway. The company plans to start human clinical trials in the summer of 2023 and gain approval for market launch in 2027.
Dr. Holmes has a doctorate in medical engineering, and Nanochon Co-founder and CTO Nathan Castro, Ph.D., has a doctorate in tissue engineering and regenerative medicine. Their research in biomaterials and 3D printing led them to develop their material and then found the company in 2016.
What interested you in this intersection of 3D printing and nanomaterials?
Dr. Holmes: My work at the time focused on osteochondral regeneration, which is the interface between bone and surface cartilage in a joint. I thought we could 3D-print different materials or structures. Certainly, other people have done similar work, but we saw the potential of an initial first-use case in printing a bone structure that integrated well with a disparate cartilage structure.
What I found and what my partner in the business found is that 3D printing is an effective way to create complex three-dimensional structures and disparate structures within the same build. Even though there are other ways to manufacture these structures, 3D printing is a fast, effective way to do it and offers an opportunity to scale in a manufacturing setting.
3D printing introduces complexity where traditional manufacturing or traditional material synthesis processes fail. What interests us is the ability to continue this approach of biomimicry, of creating structures that mimic what’s in the body, and that, in turn, catalyze a response of cells and create the tissue you want.
We can take these tissue engineering approaches and elevate them to a level where you get the same effect at a clinically relevant volume and in a living body.
Numerous emergent products are using 3D printing and biomaterials. Why did you decide to pursue cartilage replacement and repair?
Dr. Holmes: Cartilage degeneration is an issue that virtually everyone will experience in their lifetime. It is a clinically relevant problem that needs to be addressed adequately for patients. The clinical standard is still mitigation and delay. Can you stop the degeneration, or can you slow it down to the point where someone may not need a knee replacement? Cartilage degeneration has huge long-term health implications.
This also addresses the care of younger folks. The sports medicine market is growing 8% annually and is primarily driven by knee treatments.
From a technical standpoint, cartilage repair is elusive. In a purely academic setting, it continues to be a very challenging but exciting problem.
It was the confluence of those two reasons. You need good science to solve the problem, but once you put in the effort and time to solve it, there is a big opportunity and a big need for patients.
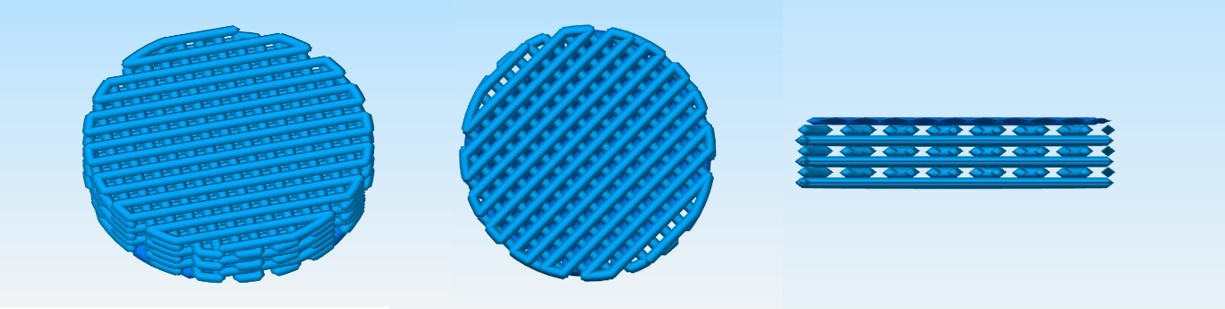
Nanochon’s Chondrograft is one part orthopedic load-bearing implant, one part tissue growth scaffold. The company’s implant is made of a 3D-printed nylon-based material.
How would you describe your Chondrograft technology?
Dr. Holmes: There are two things I ascribe to it. First, is the newness of its material and approach. Many cartilage implants are repackaged or repurposed technologies that failed on the first attempt at development or commercialization, or they were used in a certain way and then repurposed for cartilage resurfacing. Our technology truly is new material.
Additionally, many companies have used 3D printing to create a more viable suspension for live cells. That’s not what we’re doing. Our application of 3D printing as a better core structure for cartilage has not been done before, to my knowledge.
Second, in terms of surgical workflow, our Chondrograft is less invasive, meaning surgeons apply it arthroscopically while removing less healthy tissue. The 3D-printed structure also allows for flexible forming and molding of the implant. While it has a defined and mechanically stable shape, surgeons can still bend it and apply it through an incision or a working channel. The surgeons I talk to care about being able to move the implant through a fully arthroscopic workflow. Companies continually fail to address that factor, and it boggles my mind.
We aren’t seeding the technology with a cell or tissue sample. It’s an off-the-shelf product that surgeons can grab and use as they want, which hasn’t yet been done effectively.
We’ve developed a technology and a packaging that checks all the boxes, and you need to check all the boxes in order to gain adoption.
What material are you using?
Dr. Holmes: The main component is a formulation of nylon that is highly micro- and nano-porous, which gives the material initial bioactivity.
3D printing is an effective way for us to create larger, well-organized core structures that facilitate rapid integration. Our preclinical studies have shown that at a longer time point, high-quality hyaline cartilage is achieved. Even at a shorter time point, extremely rapid non-inflammatory integration is achieved. The body responds to the material purely as a matrix, which is why our product works so well.
What we like about the material is that it’s a great substrate for stem cell growth. It’s non-inflammatory, non-immune triggering. Then we can 3D-print it into a larger structure, to get rapid integration with native cell populations.
What type of 3D printing are you using?
Dr. Holmes: We didn’t want to create our own process or become a 3D-printer company. Our material is compatible with an FDM-style printer. We’re using a production quality, high-tolerance, high-volume printer that provides the accuracy we want and need, with very little post-processing required.
Do you see this as a platform technology?
Dr. Holmes: Absolutely. Looking at other large joints, lesions occur in the ankle, hip and shoulder. There are also opportunities to continue to tailor the project. We’ve received interest from investors in small joint extremities, hand and wrist procedures, and foot surgery. I also think there’s an opportunity for soft tissue reconstruction in the craniomaxillofacial space.
CL
Carolyn LaWell is ORTHOWORLD's Chief Content Officer. She joined ORTHOWORLD in 2012 to oversee its editorial and industry education. She previously served in editor roles at B2B magazines and newspapers.




